Yeast nuclei display prominent centromere clustering that is reduced in nondividing cells and in meiotic prophase
- PMID: 9531545
- PMCID: PMC2132713
- DOI: 10.1083/jcb.141.1.21
Yeast nuclei display prominent centromere clustering that is reduced in nondividing cells and in meiotic prophase
Abstract
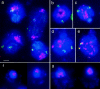
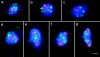
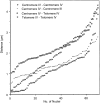
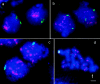
Chromosome arrangement in spread nuclei of the budding yeast, Saccharomyces cerevisiae was studied by fluorescence in situ hybridization with probes to centromeres and telomeric chromosome regions. We found that during interphase centromeres are tightly clustered in a peripheral region of the nucleus, whereas telomeres tend to occupy the area outside the centromeric domain. In vigorously growing cultures, centromere clustering occurred in approximately 90% of cells and it appeared to be maintained throughout interphase. It was reduced when cells were kept under stationary conditions for an extended period. In meiosis, centromere clusters disintegrated before the emergence of the earliest precursors of the synaptonemal complex. Evidence for the contribution of centromere clustering to other aspects of suprachromosomal nuclear order, in particular the vegetative association of homologous chromosomes, is provided, and a possible supporting role in meiotic homology searching is discussed.
Figures
References
-
- Aragón-Alcaide L, Reader S, Beven A, Shaw P, Miller T, Moore G. Association of homologous chromosomes during floral development. Curr Biol. 1997;7:905–908. - PubMed
-
- Avivi L, Feldman M. Arrangement of chromosomes in the interphase nucleus of plants. Hum Genet. 1980;55:281–295. - PubMed
-
- Bartholdi MF. Nuclear distribution of centromeres during the cell cycle of human diploid fibroblasts. J Cell Sci. 1991;99:255–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

