Cargo selection by the COPII budding machinery during export from the ER
- PMID: 9531548
- PMCID: PMC2132735
- DOI: 10.1083/jcb.141.1.61
Cargo selection by the COPII budding machinery during export from the ER
Abstract
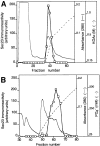
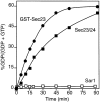
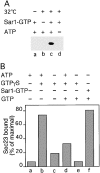
Cargo is selectively exported from the ER in COPII vesicles. To analyze the role of COPII in selective transport from the ER, we have purified components of the mammalian COPII complex from rat liver cytosol and then analyzed their role in cargo selection and ER export. The purified mammalian Sec23-24 complex is composed of an 85-kD (Sec23) protein and a 120-kD (Sec24) protein. Although the Sec23-24 complex or the monomeric Sec23 subunit were found to be the minimal cytosolic components recruited to membranes after the activation of Sar1, the addition of the mammalian Sec13-31 complex is required to complete budding. To define possible protein interactions between cargo and coat components, we recruited either glutathione-S-transferase (GST)-tagged Sar1 or GST- Sec23 to ER microsomes. Subsequently, we solubilized and reisolated the tagged subunits using glutathione-Sepharose beads to probe for interactions with cargo. We find that activated Sar1 in combination with either Sec23 or the Sec23-24 complex is necessary and sufficient to recover with high efficiency the type 1 transmembrane cargo protein vesicular stomatitis virus glycoprotein in a detergent-soluble prebudding protein complex that excludes ER resident proteins. Supplementing these minimal cargo recruitment conditions with the mammalian Sec13-31 complex leads to export of the selected cargo into COPII vesicles. The ability of cargo to interact with a partial COPII coat demonstrates that these proteins initiate cargo sorting on the ER membrane before budding and establishes the role of GTPase-dependent coat recruitment in cargo selection.
Figures










References
-
- Aridor M, Balch WE. Principles of selective transport: coat complexes hold the key. Trends Cell Biol. 1996a;6:315–320. - PubMed
-
- Aridor M, Balch WE. Timing is everything. Nature. 1996b;383:220–221. - PubMed
-
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

