Dynamin-mediated internalization of caveolae
- PMID: 9531550
- PMCID: PMC2132718
- DOI: 10.1083/jcb.141.1.85
Dynamin-mediated internalization of caveolae
Abstract
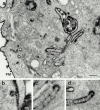
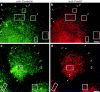
The dynamins comprise an expanding family of ubiquitously expressed 100-kD GTPases that have been implicated in severing clathrin-coated pits during receptor-mediated endocytosis. Currently, it is unclear whether the different dynamin isoforms perform redundant functions or participate in distinct endocytic processes. To define the function of dynamin II in mammalian epithelial cells, we have generated and characterized peptide-specific antibodies to domains that either are unique to this isoform or conserved within the dynamin family. When microinjected into cultured hepatocytes these affinity-purified antibodies inhibited clathrin-mediated endocytosis and induced the formation of long plasmalemmal invaginations with attached clathrin-coated pits. In addition, clusters of distinct, nonclathrin-coated, flask-shaped invaginations resembling caveolae accumulated at the plasma membrane of antibody-injected cells. In support of this, caveola-mediated endocytosis of labeled cholera toxin B was inhibited in antibody-injected hepatocytes. Using immunoisolation techniques an anti-dynamin antibody isolated caveolar membranes directly from a hepatocyte postnuclear membrane fraction. Finally, double label immunofluorescence microscopy revealed a striking colocalization between dynamin and the caveolar coat protein caveolin. Thus, functional in vivo studies as well as ultrastructural and biochemical analyses indicate that dynamin mediates both clathrin-dependent endocytosis and the internalization of caveolae in mammalian cells.
Figures
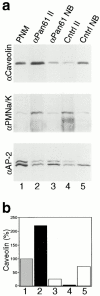

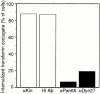
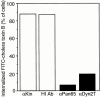

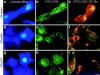


References
-
- Anderson RGW. Plasmalemmal caveolae and GPI-anchored membrane proteins. Curr Opin Cell Biol. 1993b;5:647–652. - PubMed
-
- Anderson RGW. Potocytosis of small molecules and ions by caveolae. Trends Cell Biol. 1993c;3:69–72. - PubMed
-
- Anderson RGW, Brown MS, Goldstein JL. Role of the coated endocytic vesicle in the uptake of receptor bound low density lipoprotein in human fibroblasts. Cell. 1977;10:351–364. - PubMed
-
- Anderson RGW, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

