Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome
- PMID: 9531556
- PMCID: PMC2132723
- DOI: 10.1083/jcb.141.1.163
Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome
Abstract
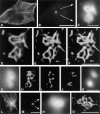
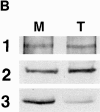
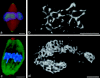
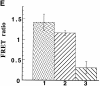
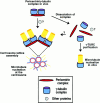
Pericentrin and gamma-tubulin are integral centrosome proteins that play a role in microtubule nucleation and organization. In this study, we examined the relationship between these proteins in the cytoplasm and at the centrosome. In extracts prepared from Xenopus eggs, the proteins were part of a large complex as demonstrated by sucrose gradient sedimentation, gel filtration and coimmunoprecipitation analysis. The pericentrin-gamma-tubulin complex was distinct from the previously described gamma-tubulin ring complex (gamma-TuRC) as purified gamma-TuRC fractions did not contain detectable pericentrin. When assembled at the centrosome, the two proteins remained in close proximity as shown by fluorescence resonance energy transfer. The three- dimensional organization of the centrosome-associated fraction of these proteins was determined using an improved immunofluorescence method. This analysis revealed a novel reticular lattice that was conserved from mammals to amphibians, and was organized independent of centrioles. The lattice changed dramatically during the cell cycle, enlarging from G1 until mitosis, then rapidly disassembling as cells exited mitosis. In cells colabeled to detect centrosomes and nucleated microtubules, lattice elements appeared to contact the minus ends of nucleated microtubules. Our results indicate that pericentrin and gamma-tubulin assemble into a unique centrosome lattice that represents the higher-order organization of microtubule nucleating sites at the centrosome.
Figures
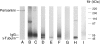








References
-
- Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991;349:694–697. - PubMed
-
- Albers K, Fuchs E. The molecular biology of intermediate filaments. Int Rev Cytol. 1992;134:243–279. - PubMed
-
- Archer J, Solomon F. Deconstructing the microtubule-organizing center. Cell. 1994;76:589–592. - PubMed
-
- Blomberg, M., and S.J. Doxsey. 1998. Rapid isolation of centrosomes. Methods Enzymol. In press. - PubMed
-
- Brown R, Doxsey SJ, Hong-Brown L, Martin RL, Welsh W. Molecular chaperones and the centrosome: a role for TCP-1 in microtubule nucleation. J Biol Chem. 1996;271:824–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

