Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator
- PMID: 9531563
- PMCID: PMC2132728
- DOI: 10.1083/jcb.141.1.255
Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator
Abstract
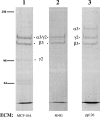
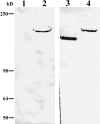
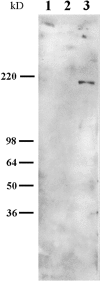
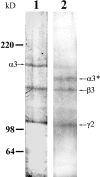
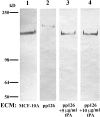
The laminin-5 component of the extracellular matrices of certain cultured cells such as the rat epithelial cell line 804G and the human breast epithelial cell MCF-10A is capable of nucleating assembly of cell- matrix adhesive devices called hemidesmosomes when other cells are plated upon them. These matrices also impede cell motility. In contrast, cells plated onto the laminin-5-rich matrices of pp126 epithelial cells fail to assemble hemidesmosomes and are motile. To understand these contradictory phenomena, we have compared the forms of heterotrimeric laminin-5 secreted by 804G and MCF-10A cells with those secreted by pp126 cells, using a panel of laminin-5 subunit-specific antibodies. The alpha3 subunit of laminin-5 secreted by pp126 cells migrates at 190 kD, whereas that secreted by 804G and MCF-10A cells migrates at 160 kD. The pp126 cell 190-kD alpha3 chain of laminin-5 can be specifically proteolyzed by plasmin to a 160-kD species at enzyme concentrations that do not apparently effect the laminin-5 beta and gamma chains. After plasmin treatment, pp126 cell laminin-5 not only impedes cell motility but also becomes competent to nucleate assembly of hemidesmosomes. The possibility that plasmin may play an important role in processing laminin-5 subunits is supported by immunofluorescence analyses that demonstrate colocalization of laminin-5 and plasminogen in the extracellular matrix of MCF-10A and pp126 cells. Whereas tissue-type plasminogen activator (tPA), which converts plasminogen to plasmin, codistributes with laminin-5 in MCF-10A matrix, tPA is not present in pp126 extracellular matrix. Treatment of pp126 laminin-5-rich extracellular matrix with exogenous tPA results in proteolysis of the laminin-5 alpha3 chain from 190 to 160 kD. In addition, plasminogen and tPA bind laminin-5 in vitro. In summary, we provide evidence that laminin-5 is a multifunctional protein that can act under certain circumstances as a motility and at other times as an adhesive factor. In cells in culture, this functional conversion appears dependent upon and is regulated by tPA and plasminogen.
Figures






References
-
- Baker SE, Hopkinson SB, Fitchmun M, Andreason GL, Frasier F, Plopper G, Quaranta V, Jones JCR. Laminin-5 and hemidesmosomes: role of the α3 subunit in hemidesmosome stability and assembly. J Cell Sci. 1996a;109:2509–2520. - PubMed
-
- Baker SE, DiPasquale A, Stock EL, Plopper G, Quaranta V, Fitchmun M, Jones JCR. Morphogenetic effects and utility in organ culture of a soluble laminin variant, laminin-5. Exp Cell Res. 1996b;228:262–270. - PubMed
-
- Borradori L, Sonnenberg A. Hemidesmosomes: role in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647–656. - PubMed
-
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell. 1991;65:599–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

