The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes
- PMID: 9531565
- PMCID: PMC2132720
- DOI: 10.1083/jcb.141.1.281
The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes
Abstract
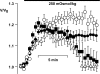
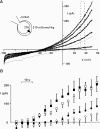
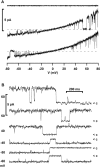
Osmotic cell swelling activates Cl- channels to achieve anion efflux. In this study, we find that both the tyrosine kinase inhibitor herbimycin A and genetic knockout of p56lck, a src-like tyrosine kinase, block regulatory volume decrease (RVD) in a human T cell line. Activation of a swelling-activated chloride current (ICl-swell) by osmotic swelling in whole-cell patch-clamp experiments is blocked by herbimycin A and lavendustin. Osmotic activation of ICl-swell is defective in p56lck-deficient cells. Retransfection of p56lck restores osmotic current activation. Furthermore, tyrosine kinase activity is sufficient for activation of ICl-swell. Addition of purified p56lck to excised patches activates an outwardly rectifying chloride channel with 31 pS unitary conductance. Purified p56lck washed into the cytoplasm activates ICl-swell in native and p56lck-deficient cells even when hypotonic intracellular solutions lead to cell shrinkage. When whole-cell currents are activated either by swelling or by p56lck, slow single-channel gating events can be observed revealing a unitary conductance of 25-28 pS. In accordance with our patch-clamp data, osmotic swelling increases activity of immunoprecipitated p56lck. We conclude that osmotic swelling activates ICl-swell in lymphocytes via the tyrosine kinase p56lck.
Figures



References
-
- August A, Dupont B. Activation of extracellular signal-regulated protein kinase (ERK/MAP kinase) following CD28 cross-linking: activation in cells lacking p56lck. Tissue Antigens. 1995;46:155–162. - PubMed
-
- Cahalan M.D., and R.S. Lewis. 1988. Role of potassium and chloride channels in volume regulation by T lymphocytes. In Cell Physiology of Blood. R.B. Gunn, and J.C. Parker, editors. Rockefeller University Press, New York. 281–301. - PubMed
-
- Deutsch C, Lee SC. Cell volume regulation in lymphocytes. Renal Physiol Biochem. 1988;3:260–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

