Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration
- PMID: 9536037
- PMCID: PMC35027
- DOI: 10.1104/pp.116.4.1209
Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration
Abstract
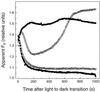
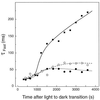
We investigated the relationship between nonphotochemical plastoquinone reduction and chlororespiration in leaves of growth-chamber-grown sunflower (Helianthus annuus L.). Following a short induction period, leaves of previously illuminated sunflower showed a substantially increased level of minimal fluorescence following a light-to-dark transition. This increase in minimal fluorescence was reversed by far-red illumination, inhibited by rotenone or photooxidative methyl viologen treatment, and stimulated by fumigation with CO. Using flash-induced electrochromic absorption-change measurements, we observed that the capacity of sunflower to reduce plastoquinone in the dark influenced the activation state of the chloroplast ATP synthase, although chlororespiratory transmembrane electrochemical potential formation alone does not fully explain our observations. We have added several important new observations to the work of others, forming, to our knowledge, the first strong experimental evidence that chlororespiratory, nonphotochemical plastoquinone reduction and plastoquinol oxidation occur in the chloroplasts of higher plants. We have introduced procedures for monitoring and manipulating chlorores-piratory activity in leaves that will be important in subsequent work aimed at defining the pathway and function of this dark electron flux in higher plant chloroplasts.
Figures




References
-
- Aristarkhov AI, Nikandrov VV, Krasnovskii AA. Ascorbate permeability of chloroplast thylakoid membrane; reduction of plastoquinone and cytochrome f. Biokhimiya. 1987;52:1729–1808.
-
- Asada K, Heber U, Schreiber U. Pool size of electrons that can be donated to P700+, as determined in intact leaves: donation of P700+ from stromal components via the intersystem chain. Plant Cell Physiol. 1992;33:927–932.
-
- Bendall DS, Manasse RS. Cyclic photophosphorylation and electron transport. Biochim Biophys Acta. 1995;1229:23–38.
-
- Bennoun P. Effects of mutations and of ionophores on chlororespiration in Chlamydomonas reinhardtii. FEBS Lett. 1993;156:363–365.
LinkOut - more resources
Full Text Sources

