Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga dunaliella bardawil
- PMID: 9536040
- PMCID: PMC35030
- DOI: 10.1104/pp.116.4.1239
Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga dunaliella bardawil
Abstract
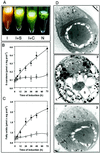
Under stress conditions such as high light intensity or nutrient starvation, cells of the unicellular alga Dunaliella bardawil overproduce beta-carotene, which is accumulated in the plastids in newly formed triacylglycerol droplets. We report here that the formation of these sequestering structures and beta-carotene are interdependent. When the synthesis of triacylglycerol is blocked, the overproduction of beta-carotene is also inhibited. During overproduction of beta-carotene no up-regulation of phytoene synthase or phytoene desaturase is observed on the transcriptional or translational level, whereas at the same time acetyl-CoA carboxylase, the key regulatory enzyme of acyl lipid biosynthesis, is increased, at least in its enzymatic activity. We conclude that under normal conditions the carotenogenic pathway is not maximally active and may be appreciably stimulated in the presence of sequestering structures, creating a plastid-localized sink for the end product of the carotenoid biosynthetic pathway.
Figures





References
-
- Al-Babili S, v Lintig J, Haubruck H, Beyer P (1996) A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J 9: 601–612 - PubMed
-
- Bejarano ER, Parra F, Murillo FJ, Cerdá-Olmedo E. End-product regulation of carotenogenesis in Phycomyces. Arch Microbiol. 1988;150:209–214.
-
- Ben Amotz A. Adaptation of the unicellular alga Dunaliella parva to saline environment. J Phycol. 1975;11:50–54.
LinkOut - more resources
Full Text Sources

