Biological activity of reducing-end-derivatized oligogalacturonides in tobacco tissue cultures
- PMID: 9536045
- PMCID: PMC35035
- DOI: 10.1104/pp.116.4.1289
Biological activity of reducing-end-derivatized oligogalacturonides in tobacco tissue cultures
Abstract
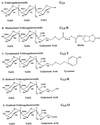
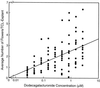
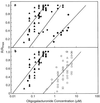
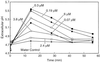
The biological activity of reducing-end-modified oligogalacturonides was quantified in four tobacco (Nicotiana tabacum) tissue culture bioassays. The derivatives used were oligogalacturonides with the C-1 of their reducing end (a) covalently linked to a biotin hydrazide, (b) covalently linked to tyramine, (c) chemically reduced to a primary alcohol, or (d) enzymatically oxidized to a carboxylic acid. These derivatives were tested for their ability to (a) alter morphogenesis of N. tabacum cv Samsun thin cell-layer explants, (b) elicit extracellular alkalinization by suspension-cultured cv Samsun cells, (c) elicit extracellular alkalinization by suspension-cultured N. tabacum cv Xanthi cells, and (d) elicit H2O2 accumulation in the cv Xanthi cells. In all four bioassays, each of the derivatives had reduced biological activity compared with the corresponding underivatized oligogalacturonides, demonstrating that the reducing end is a key element for the recognition of oligogalacturonides in these systems. However, the degree of reduction in biological activity depends on the tissue culture system used and on the nature of the specific reducing-end modification. These results suggest that oligogalacturonides are perceived differently in each tissue culture system.
Figures



References
-
- Basse CW, Fath A, Boller T. High affinity binding of a glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors. J Biol Chem. 1993;268:14724–14731. - PubMed
-
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes. Competitive inhibition of binding by derivatives of chitin fragments and a nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. - PubMed
-
- Bellincampi D, Salvi G, De Lorenzo G, Cervone F, Marfà V, Eberhard S, Darvill A, Albersheim P. Oligogalacturonides inhibit the formation of roots on tobacco explants. Plant J. 1993;4:207–213.
-
- Bishop PD, Pearce G, Bryant JE, Ryan CA. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984;259:13172–13177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

