Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant
- PMID: 9536049
- PMCID: PMC35039
- DOI: 10.1104/pp.116.4.1323
Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant
Abstract
The induction of the sucrose synthase (SuSy) gene (SuSy) by low O2, low temperature, and limiting carbohydrate supply suggested a role in carbohydrate metabolism under stress conditions. The isolation of a maize (Zea mays L.) line mutant for the two known SuSy genes but functionally normal showed that SuSy activity might not be required for aerobic growth and allowed the possibility of investigating its importance during anaerobic stress. As assessed by root elongation after return to air, hypoxic pretreatment improved anoxic tolerance, in correlation with the number of SuSy genes and the level of SuSy expression. Furthermore, root death in double-mutant seedlings during anoxic incubation could be attributed to the impaired utilization of sucrose (Suc). Collectively, these data provide unequivocal evidence that Suc is the principal C source and that SuSy is the main enzyme active in Suc breakdown in roots of maize seedlings deprived of O2. In this situation, SuSy plays a critical role in anoxic tolerance.
Figures


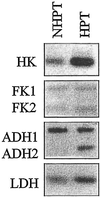


References
-
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Chourey PS. Genetic control of sucrose synthetase in maize endosperm. Mol Gen Genet. 1981;184:372–376.
-
- Chourey PS. Recombinants lacking in detectable levels of both sucrose synthases are functionally normal. Maize Genet Coop Newslett. 1988;62:62–63.
LinkOut - more resources
Full Text Sources
Research Materials

