Soybean lipoxygenase-1 oxidizes 3Z-nonenal. A route to 4s-hydroperoxy-2e-nonenal and related products
- PMID: 9536053
- PMCID: PMC35043
Soybean lipoxygenase-1 oxidizes 3Z-nonenal. A route to 4s-hydroperoxy-2e-nonenal and related products
Abstract
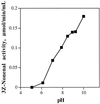
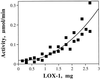
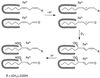
In previous work with soybean (Glycine max), it was reported that the initial product of 3Z-nonenal (NON) oxidation is 4-hydroperoxy-2E-nonenal (4-HPNE). 4-HPNE can be converted to 4-hydroxy-2E-nonenal by a hydroperoxide-dependent peroxygenase. In the present work we have attempted to purify the 4-HPNE-producing oxygenase from soybean seed. Chromatography on various supports had shown that O2 uptake with NON substrate consistently coincided with lipoxygenase (LOX)-1 activity. Compared with oxidation of LOX's preferred substrate, linoleic acid, the activity with NON was about 400- to 1000-fold less. Rather than obtaining the expected 4-HPNE, 4-oxo-2E-nonenal was the principal product of NON oxidation, presumably arising from the enzyme-generated alkoxyl radical of 4-HPNE. In further work a precipitous drop in activity was noted upon dilution of LOX-1 concentration; however, activity could be enhanced by spiking the reaction with 13S-hydroperoxy-9Z, 11E-octadecadienoic acid. Under these conditions the principal product of NON oxidation shifted to the expected 4-HPNE. 4-HPNE was demonstrated to be 83% of the 4S-hydroperoxy-stereoisomer. Therefore, LOX-1 is also a 3Z-alkenal oxygenase, and it exerts the same stereospecificity of oxidation as it does with polyunsaturated fatty acids. Two other LOX isozymes of soybean seed were also found to oxidize NON to 4-HPNE with an excess of 4S-hydroperoxy-stereoisomer.
Figures





References
-
- Axelrod B, Cheesbrough TM, Laakso S. Lipoxygenase from soybeans. Methods Enzymol. 1981;71:441–451.
-
- Blée E, Schuber F. Stereochemistry of the epoxidation of fatty acids catalyzed by soybean peroxygenase. Biochem Biophys Res Commun. 1990;173:1354–1360. - PubMed
-
- Blée E, Schuber F. Regio- and enantioselectivity of soybean fatty acid epoxide hydrolase. J Biol Chem. 1992;267:11881–11887. - PubMed
-
- Carini R, Bellomo G, Paradisi L, Dianzani MU, Albano E. 4-Hydroxynonenal triggers Ca2+ influx in isolated rat hepatocytes. Biochem Biophys Res Commun. 1996;218:772–776. - PubMed
-
- Christopher JP, Pistorius EK, Axelrod B. Isolation of an isozyme of soybean lipoxygenase. Biochim Biophys Acta. 1970;198:12–19. - PubMed
LinkOut - more resources
Full Text Sources
