Characterization of a gene for spinach CAP160 and expression of two spinach cold-acclimation proteins in tobacco
- PMID: 9536054
- PMCID: PMC35044
- DOI: 10.1104/pp.116.4.1367
Characterization of a gene for spinach CAP160 and expression of two spinach cold-acclimation proteins in tobacco
Abstract
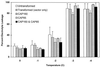
The cDNA sequence for CAP160, an acidic protein previously linked with cold acclimation in spinach (Spinacia oleracea L.), was characterized and found to encode a novel acidic protein of 780 amino acids having very limited homology to a pair of Arabidopsis thaliana stress-regulated proteins, rd29A and rd29B. The lack of similarity in the structural organization of the spinach and Arabidopsis genes highlights the absence of a high degree of conservation of this cold-stress gene across taxonomic boundaries. The protein has several unique motifs that may relate to its function during cold stress. Expression of the CAP160 mRNA was increased by low-temperature exposure and water stress in a manner consistent with a probable function during stresses that involve dehydration. The coding sequences for CAP160 and CAP85, another spinach cold-stress protein, were introduced into tobacco (Nicotiana tabacum) under the control of the 35S promoter using Agrobacterium tumefaciens-based transformation. Tobacco plants expressing the proteins individually or coexpressing both proteins were evaluated for relative freezing-stress tolerance. The killing temperature for 50% of the cells of the transgenic plants was not different from that of the wild-type plants. As determined by a more sensitive time/temperature kinetic study, plants expressing the spinach proteins had slightly lower levels of electrolyte leakage than wild-type plants, indicative of a small reduction of freezing-stress injury. Clearly, the heterologous expression of two cold-stress proteins had no profound influence on stress tolerance, a result that is consistent with the quantitative nature of cold-stress-tolerance traits.
Figures







References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1991) Current Protocols in Molecular Biology, Vol 2. Wiley, New York
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

