Polypeptides of the maize amyloplast stroma. Stromal localization of starch-biosynthetic enzymes and identification of an 81-kilodalton amyloplast stromal heat-shock cognate
- PMID: 9536063
- PMCID: PMC35053
- DOI: 10.1104/pp.116.4.1451
Polypeptides of the maize amyloplast stroma. Stromal localization of starch-biosynthetic enzymes and identification of an 81-kilodalton amyloplast stromal heat-shock cognate
Abstract
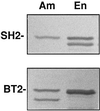
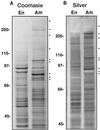
In the developing endosperm of monocotyledonous plants, starch granules are synthesized and deposited within the amyloplast. A soluble stromal fraction was isolated from amyloplasts of immature maize (Zea mays L.) endosperm and analyzed for enzyme activities and polypeptide content. Specific activities of starch synthase and starch-branching enzyme (SBE), but not the cytosolic marker alcohol dehydrogenase, were strongly enhanced in soluble amyloplast stromal fractions relative to soluble extracts obtained from homogenized kernels or endosperms. Immunoblot analysis demonstrated that starch synthase I, SBEIIb, and sugary1, the putative starch-debranching enzyme, were each highly enriched in the amyloplast stroma, providing direct evidence for the localization of starch-biosynthetic enzymes within this compartment. Analysis of maize mutants shows the deficiency of the 85-kD SBEIIb polypeptide in the stroma of amylose extender cultivars and that the dull mutant lacks a >220-kD stromal polypeptide. The stromal fraction is distinguished by differential enrichment of a characteristic group of previously undocumented polypeptides. N-terminal sequence analysis revealed that an abundant 81-kD stromal polypeptide is a member of the Hsp70 family of stress-related proteins. Moreover, the 81-kD stromal polypeptide is strongly recognized by antibodies specific for an Hsp70 of the chloroplast stroma. These findings are discussed in light of implications for the correct folding and assembly of soluble, partially soluble, and granule-bound starch-biosynthetic enzymes during import into the amyloplast.
Figures







Similar articles
-
Identification of the maize amyloplast stromal 112-kD protein as a plastidic starch phosphorylase.Plant Physiol. 2001 Jan;125(1):351-9. doi: 10.1104/pp.125.1.351. Plant Physiol. 2001. PMID: 11154342 Free PMC article.
-
Allelic variants of the amylose extender mutation of maize demonstrate phenotypic variation in starch structure resulting from modified protein-protein interactions.J Exp Bot. 2012 Feb;63(3):1167-83. doi: 10.1093/jxb/err341. Epub 2011 Nov 25. J Exp Bot. 2012. PMID: 22121198 Free PMC article.
-
The amylose extender mutant of maize conditions novel protein-protein interactions between starch biosynthetic enzymes in amyloplasts.J Exp Bot. 2009;60(15):4423-40. doi: 10.1093/jxb/erp297. Epub 2009 Oct 4. J Exp Bot. 2009. PMID: 19805395
-
Current models for starch synthesis and the sugary enhancer1 (se1) mutation in Zea mays.Plant Physiol Biochem. 2004 Jun;42(6):457-64. doi: 10.1016/j.plaphy.2004.05.008. Plant Physiol Biochem. 2004. PMID: 15246058 Review.
-
Starch synthesis in developing pea embryos.New Phytol. 1992 Sep;122(1):21-33. doi: 10.1111/j.1469-8137.1992.tb00049.x. New Phytol. 1992. PMID: 33874037 Review.
Cited by
-
The impact of the indica rice SSIIa allele on the apparent high amylose starch from rice grain with downregulated japonica SBEIIb.Theor Appl Genet. 2020 Oct;133(10):2961-2974. doi: 10.1007/s00122-020-03649-2. Epub 2020 Jul 10. Theor Appl Genet. 2020. PMID: 32651668
-
Proteome Profile of Starch Granules Purified from Rice (Oryza sativa) Endosperm.PLoS One. 2016 Dec 19;11(12):e0168467. doi: 10.1371/journal.pone.0168467. eCollection 2016. PLoS One. 2016. PMID: 27992503 Free PMC article.
-
Purification and molecular genetic characterization of ZPU1, a pullulanase-type starch-debranching enzyme from maize.Plant Physiol. 1999 Jan;119(1):255-66. doi: 10.1104/pp.119.1.255. Plant Physiol. 1999. PMID: 9880368 Free PMC article.
-
Three isoforms of isoamylase contribute different catalytic properties for the debranching of potato glucans.Plant Cell. 2003 Jan;15(1):133-49. doi: 10.1105/tpc.006635. Plant Cell. 2003. PMID: 12509527 Free PMC article.
-
Using the dominant mutation gene Ae1-5180 (amylose extender) to develop high-amylose maize.Mol Breed. 2022 Sep 17;42(10):57. doi: 10.1007/s11032-022-01323-7. eCollection 2022 Oct. Mol Breed. 2022. PMID: 37313014 Free PMC article.
References
-
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

