Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana
- PMID: 9536070
- PMCID: PMC35060
- DOI: 10.1104/pp.116.4.1515
Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana
Abstract
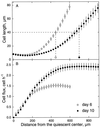
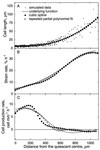
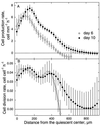
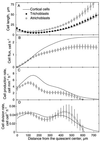
To investigate the relation between cell division and expansion in the regulation of organ growth rate, we used Arabidopsis thaliana primary roots grown vertically at 20 degreesC with an elongation rate that increased steadily during the first 14 d after germination. We measured spatial profiles of longitudinal velocity and cell length and calculated parameters of cell expansion and division, including rates of local cell production (cells mm-1 h-1) and cell division (cells cell-1 h-1). Data were obtained for the root cortex and also for the two types of epidermal cell, trichoblasts and atrichoblasts. Accelerating root elongation was caused by an increasingly longer growth zone, while maximal strain rates remained unchanged. The enlargement of the growth zone and, hence, the accelerating root elongation rate, were accompanied by a nearly proportionally increased cell production. This increased production was caused by increasingly numerous dividing cells, whereas their rates of division remained approximately constant. Additionally, the spatial profile of cell division rate was essentially constant. The meristem was longer than generally assumed, extending well into the region where cells elongated rapidly. In the two epidermal cell types, meristem length and cell division rate were both very similar to that of cortical cells, and differences in cell length between the two epidermal cell types originated at the apex of the meristem. These results highlight the importance of controlling the number of dividing cells, both to generate tissues with different cell lengths and to regulate the rate of organ enlargement.
Figures

References
-
- Balodis VA, Ivanov VB. Proliferation of root cells in the basal part of meristem and apical part of elongation zone. Tsitologia. 1970;12:983–991. (in Russian)
-
- Balus̆ka F, Barlow PW, Kubica S̆. Importance of the post-mitotic isodiametric growth (PIG) region for growth and development of roots. Plant Soil. 1994;167:31–41.
-
- Barlow PW. Cell growth in the absence of division in a root meristem. Planta. 1969;88:215–223. - PubMed
-
- Barlow PW. Cellular packets, cell division and morphogenesis in the primary root meristem of Zea mays L. New Phytol. 1987;105:27–56. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

