Evidence for 1-(Malonylamino)cyclopropane-1-carboxylic acid being the major conjugate of aminocyclopropane-1-carboxylic acid in tomato fruit
- PMID: 9536071
- PMCID: PMC35061
- DOI: 10.1104/pp.116.4.1527
Evidence for 1-(Malonylamino)cyclopropane-1-carboxylic acid being the major conjugate of aminocyclopropane-1-carboxylic acid in tomato fruit
Abstract
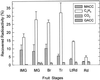
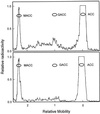
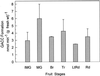
Tomato (Lycopersicon esculentum Miller) fruit discs fed with [2, 3-14C]1-aminocyclopropane-1-carboxylic acid (ACC) formed 1-malonyl-ACC (MACC) as the major conjugate of ACC in fruit throughout all ripening stages, from immature-green through the red-ripe stage. Another conjugate of ACC, gamma-glutamyl-ACC (GACC), was formed only in mature-green fruit in an amount about 10% of that of MACC; conjugation of ACC into GACC was not detected in fruits at other ripening stages. No GACC formation was observed from etiolated mung bean (Vigna radiata [L.] Wilczek) hypocotyls, etiolated common vetch (Vicia sativum L.) epicotyls, or pea (Pisum sativum L.) root tips, etiolated epicotyls, and green stem tissue, where active conversion of ACC into MACC was observed. GACC was, however, formed in vitro in extracts from fruit of all ripening stages. GACC formation in an extract from red fruit at pH 7.15 was only about 3% of that at pH 8.0, the pH at which most assays were run. Our present in vivo data support the previous contention that MACC is the major conjugate of ACC in plant tissues, whereas GACC is a minor, if any, conjugate of ACC. Thus, our data do not support the proposal that GACC formation could be more important than MACC formation in tomato fruit.
Figures

References
-
- Amrhein N, Breuing F, Eberle J, Skorupka H, Tophof S. The metabolism of 1-aminocyclopropane-1-carboxylic acid. In: Wareing PF, editor. Plant Growth Substances 1982. London: Academic Press; 1982. pp. 249–258.
-
- Amrhein N, Schneebeck D, Skoripka H, Tophof S, Stockigt J. Identification of a major metabolite of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid in higher plants. Naturwissenschaften. 1981;68:619–620.
-
- Goore MY, Thompson JF. γ-Glutamyl transpeptidase from kidney bean fruit. I. Purification and mechanism of action. Biochim Biophys Acta. 1967;132:15–26. - PubMed
LinkOut - more resources
Full Text Sources

