Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses
- PMID: 9536076
- PMCID: PMC35066
- DOI: 10.1104/pp.116.4.1573
Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses
Abstract
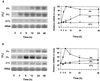
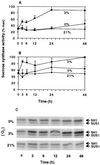
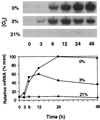
The goal of this research was to resolve the hypoxic and anoxic responses of maize (Zea mays) sucrose (Suc) synthases known to differ in their sugar regulation. The two maize Suc synthase genes, Sus1 and Sh1, both respond to sugar and O2, and recent work suggests commonalities between these signaling systems. Maize seedlings (NK508 hybrid, W22 inbred, and an isogenic sh1-null mutant) were exposed to anoxic, hypoxic, and aerobic conditions (0, 3, and 21% O2, respectively), when primary roots had reached approximately 5 cm. One-centimeter tips were excised for analysis during the 48-h treatments. At the mRNA level, Sus1 was rapidly up-regulated by hypoxia (approximately 5-fold in 6 h), whereas anoxia had less effect. In contrast, Sh1 mRNA abundance increased strongly under anoxia (approximately 5-fold in 24 h) and was much less affected by hypoxia. At the enzyme level, total Suc synthase activity rose rapidly under hypoxia but showed little significant change during anoxia. The contributions of SUS1 and SH1 activities to these responses were dissected over time by comparing the sh1-null mutant with the isogenic wild type (Sus+, Sh1+). Sh1-dependent activity contributed most markedly to a rapid protein-level response consistently observed in the first 3 h, and, subsequently, to a long-term change mediated at the level of mRNA accumulation at 48 h. A complementary midterm rise in SUS1 activity varied in duration with genetic background. These data highlight the involvement of distinctly different genes and probable signal mechanisms under hypoxia and anoxia, and together with earlier work, show parallel induction of "feast and famine" Suc synthase genes by hypoxia and anoxia, respectively. In addition, complementary modes of transcriptional and posttranscriptional regulation are implicated by these data, and provide a mechanism for sequential contributions from the Sus1 and Sh1 genes during progressive onset of naturally occurring low-O2 events.
Figures



References
-
- Bailey-Serres J, Kloeckner-Gruissem B, Freeling M. Genetic and molecular approaches to the study of the anaerobic responses and tissue specific gene expression in maize. Plant Cell Environ. 1988;11:351–357.
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
LinkOut - more resources
Full Text Sources

