The TOR (target of rapamycin) signal transduction pathway regulates the stability of translation initiation factor eIF4G in the yeast Saccharomyces cerevisiae
- PMID: 9539725
- PMCID: PMC22477
- DOI: 10.1073/pnas.95.8.4264
The TOR (target of rapamycin) signal transduction pathway regulates the stability of translation initiation factor eIF4G in the yeast Saccharomyces cerevisiae
Abstract
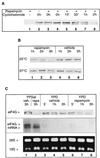
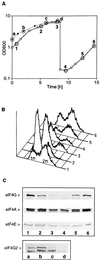
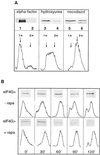
Initiation factor eIF4G is an essential protein required for initiation of mRNA translation via the 5' cap-dependent pathway. It interacts with eIF4E (the mRNA 5' cap-binding protein) and serves as an anchor for the assembly of further initiation factors. With treatment of Saccharomyces cerevisiae with rapamycin or with entry of cells into the diauxic phase, eIF4G is rapidly degraded, whereas initiation factors eIF4E and eIF4A remain stable. We propose that nutritional deprivation or interruption of the TOR signal transduction pathway induces eIF4G degradation.
Figures

References
-
- Werner-Washburne M, Braun E L, Crawford M E, Peck V M. Mol Microbiol. 1996;19:1159–1166. - PubMed
-
- Pringle J R, Hartwell L H. In: The Saccharomyces Cerevisiae Life Cycle. Strathern J N, Jones E W, Broach J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. pp. 97–142.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

