Chromophore-assisted light inactivation and self-organization of microtubules and motors
- PMID: 9539730
- PMCID: PMC22482
- DOI: 10.1073/pnas.95.8.4293
Chromophore-assisted light inactivation and self-organization of microtubules and motors
Abstract
Chromophore-assisted light inactivation (CALI) offers the only method capable of modulating specific protein activities in localized regions and at particular times. Here, we generalize CALI so that it can be applied to a wider range of tasks. Specifically, we show that CALI can work with a genetically inserted epitope tag; we investigate the effectiveness of alternative dyes, especially fluorescein, comparing them with the standard CALI dye, malachite green; and we study the relative efficiencies of pulsed and continuous-wave illumination. We then use fluorescein-labeled hemagglutinin antibody fragments, together with relatively low-power continuous-wave illumination to examine the effectiveness of CALI targeted to kinesin. We show that CALI can destroy kinesin activity in at least two ways: it can either result in the apparent loss of motor activity, or it can cause irreversible attachment of the kinesin enzyme to its microtubule substrate. Finally, we apply this implementation of CALI to an in vitro system of motor proteins and microtubules that is capable of self-organized aster formation. In this system, CALI can effectively perturb local structure formation by blocking or reducing the degree of aster formation in chosen regions of the sample, without influencing structure formation elsewhere.
Figures

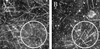

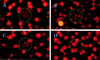
Similar articles
-
Genetically targeted chromophore-assisted light inactivation.Nat Biotechnol. 2003 Dec;21(12):1505-8. doi: 10.1038/nbt914. Epub 2003 Nov 16. Nat Biotechnol. 2003. PMID: 14625562
-
An internal motor kinesin is associated with the Golgi apparatus and plays a role in trichome morphogenesis in Arabidopsis.Mol Biol Cell. 2005 Feb;16(2):811-23. doi: 10.1091/mbc.e04-05-0400. Epub 2004 Dec 1. Mol Biol Cell. 2005. PMID: 15574882 Free PMC article.
-
Enhanced EGFP-chromophore-assisted laser inactivation using deficient cells rescued with functional EGFP-fusion proteins.Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6702-7. doi: 10.1073/pnas.0701801104. Epub 2007 Apr 9. Proc Natl Acad Sci U S A. 2007. PMID: 17420475 Free PMC article.
-
Chromophore-assisted laser inactivation--towards a spatiotemporal-functional analysis of proteins, and the ablation of chromatin, organelle and cell function.J Cell Sci. 2014 Apr 15;127(Pt 8):1621-9. doi: 10.1242/jcs.144527. J Cell Sci. 2014. PMID: 24737873 Review.
-
Chromophore-assisted laser inactivation (CALI) to elucidate cellular mechanisms of cancer.Biochim Biophys Acta. 1999 Oct 29;1424(2-3):M39-48. doi: 10.1016/s0304-419x(99)00022-0. Biochim Biophys Acta. 1999. PMID: 10528153 Review.
Cited by
-
Irradiation-induced protein inactivation reveals Golgi enzyme cycling to cell periphery.J Cell Sci. 2012 Feb 15;125(Pt 4):973-80. doi: 10.1242/jcs.094441. Epub 2012 Mar 15. J Cell Sci. 2012. PMID: 22421362 Free PMC article.
-
Ki-67 as a molecular target for therapy in an in vitro three-dimensional model for ovarian cancer.Cancer Res. 2010 Nov 15;70(22):9234-42. doi: 10.1158/0008-5472.CAN-10-1190. Epub 2010 Nov 2. Cancer Res. 2010. PMID: 21045152 Free PMC article.
-
Genetically Encoded Photosensitizer for Destruction of Protein or Cell Function.Adv Exp Med Biol. 2021;1293:265-279. doi: 10.1007/978-981-15-8763-4_16. Adv Exp Med Biol. 2021. PMID: 33398819 Review.
-
Fluorophore assisted light inactivation (FALI) of recombinant 5-HT₃A receptor constitutive internalization and function.Mol Cell Neurosci. 2011 Jun;47(2):79-92. doi: 10.1016/j.mcn.2011.02.007. Epub 2011 Feb 19. Mol Cell Neurosci. 2011. PMID: 21338684 Free PMC article.
-
Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis.Cell Prolif. 2007 Jun;40(3):422-30. doi: 10.1111/j.1365-2184.2007.00433.x. Cell Prolif. 2007. PMID: 17531085 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

