Unique molecular surface features of in vivo tolerized T cells
- PMID: 9539766
- PMCID: PMC22518
- DOI: 10.1073/pnas.95.8.4499
Unique molecular surface features of in vivo tolerized T cells
Abstract
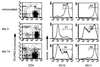
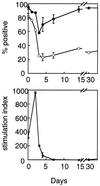
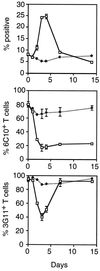
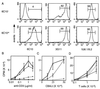
Differential expression of surface markers can frequently be used to distinguish functional subsets of T cells, yet a surface phenotype unique to T cells induced into an anergic state has not been described. Here, we report that CD4 T cells rendered anergic in vivo by superantigen can be identified by loss of the 6C10 T cell marker. Inoculation of Vbeta8.1 T cell antigen receptor (TCR) transgenic mice with a Vbeta8.1-reactive minor lymphocyte-stimulating superantigen (Mls-1(a)) induces tolerance to Mls-1(a) by clonal anergy. CD4 lymph node T cells from Mls-1(a) inoculated transgenic mice enriched for the 6C10(-) phenotype neither proliferate nor produce interleukin-2 upon TCR engagement, whereas 6C10(+) CD4 T cells retain responsiveness. Analysis of T cell memory markers demonstrate that 6C10(-) T cells remain 3G11(hi) but express heterogeneous levels of CD45RB, CD62L, CD44, and the CD69 early activation marker, suggesting that T cells at various degrees of activation can be functionally anergic. These studies demonstrate that anergic T cells can be purified based on 6C10 expression permitting examination of issues concerning biochemical and biological features specific to T cell anergy.
Figures
Similar articles
-
Naturally anergic and suppressive CD25(+)CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation.Int Immunol. 2000 Aug;12(8):1145-55. doi: 10.1093/intimm/12.8.1145. Int Immunol. 2000. PMID: 10917889
-
Phenotypic identification of the subgroups of murine T-cell receptor alphabeta+ CD4+ CD8- thymocytes and its implication in the late stage of thymocyte development.Immunology. 1999 Aug;97(4):665-71. doi: 10.1046/j.1365-2567.1999.00816.x. Immunology. 1999. PMID: 10457221 Free PMC article.
-
Anergy in peripheral memory CD4(+) T cells induced by low avidity engagement of T cell receptor.J Exp Med. 2001 Sep 17;194(6):719-31. doi: 10.1084/jem.194.6.719. J Exp Med. 2001. PMID: 11560989 Free PMC article.
-
Mechanisms of peripheral immune tolerance: conversion of the immune to the unresponsive phenotype.Immunol Res. 2003;28(3):193-9. doi: 10.1385/IR:28:3:193. Immunol Res. 2003. PMID: 14713714 Review.
-
Biochemical features of anergic T cells.Immunol Res. 1998;17(1-2):133-40. doi: 10.1007/BF02786438. Immunol Res. 1998. PMID: 9479575 Review.
Cited by
-
In vivo maintenance of T-lymphocyte unresponsiveness induced by thymic medullary epithelium requires antigen presentation by radioresistant cells.Immunology. 2003 Jan;108(1):24-31. doi: 10.1046/j.1365-2567.2003.01546.x. Immunology. 2003. PMID: 12519299 Free PMC article.
-
Anergy and suppression as coexistent mechanisms for the maintenance of peripheral T cell tolerance.Immunol Res. 2003;27(2-3):295-302. doi: 10.1385/IR:27:2-3:295. Immunol Res. 2003. PMID: 12857976 Review.
-
Suppressor T cells regulate the nonanergic cell population that remains after peripheral tolerance is induced to the Mls-1 antigen in T cell receptor Vbeta 8.1 transgenic mice.Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):13257-62. doi: 10.1073/pnas.230449097. Proc Natl Acad Sci U S A. 2000. PMID: 11069296 Free PMC article.
-
Modulation of Kv channel expression and function by TCR and costimulatory signals during peripheral CD4(+) lymphocyte differentiation.J Exp Med. 2002 Oct 7;196(7):897-909. doi: 10.1084/jem.20020381. J Exp Med. 2002. PMID: 12370252 Free PMC article.
-
3G11 expression in CD4+ T cell-mediated autoimmunity and immune tolerance.Int Immunopharmacol. 2011 May;11(5):593-6. doi: 10.1016/j.intimp.2010.11.005. Epub 2010 Nov 24. Int Immunopharmacol. 2011. PMID: 21084064 Free PMC article. Review.
References
-
- Schwartz R H. Science. 1990;248:1349–1356. - PubMed
-
- Webb S R, O’Rourke A M, Sprent J. Semin Immunol. 1992;4:329–336. - PubMed
-
- Kappler J W, Staerz U, White J, Marrack P C. Nature. 1988;332:35–40. - PubMed
-
- MacDonald H R, Schneider R, Lees R K, Howe R C, Acha-Orbea H, Festenstein H, Zinkernagel R M, Hengartner H. Nature (London) 1988;332:40–45. - PubMed
-
- Bhandoola A, Cho E A, Yui K, Saragovi H U, Greene M I, Quill H. J Immunol. 1993;151:2355–2367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

