NF-kappa B-dependent inhibition of apoptosis is essential for host cellsurvival during Rickettsia rickettsii infection
- PMID: 9539792
- PMCID: PMC22544
- DOI: 10.1073/pnas.95.8.4646
NF-kappa B-dependent inhibition of apoptosis is essential for host cellsurvival during Rickettsia rickettsii infection
Abstract
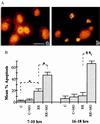
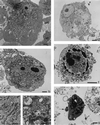
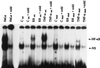
The possibility that bacteria may have evolved strategies to overcome host cell apoptosis was explored by using Rickettsia rickettsii, an obligate intracellular Gram-negative bacteria that is the etiologic agent of Rocky Mountain spotted fever. The vascular endothelial cell, the primary target cell during in vivo infection, exhibits no evidence of apoptosis during natural infection and is maintained for a sufficient time to allow replication and cell-to-cell spread prior to eventual death due to necrotic damage. Prior work in our laboratory demonstrated that R. rickettsii infection activates the transcription factor NF-kappa B and alters expression of several genes under its control. However, when R. rickettsii-induced activation of NF-kappa B was inhibited, apoptosis of infected but not uninfected endothelial cells rapidly ensued. In addition, human embryonic fibroblasts stably transfected with a superrepressor mutant inhibitory subunit Ikappa B that rendered NF-kappa B inactivatable also underwent apoptosis when infected, whereas infected wild-type human embryonic fibroblasts survived. R. rickettsii, therefore, appeared to inhibit host cell apoptosis via a mechanism dependent on NF-kappa B activation. Apoptotic nuclear changes correlated with presence of intracellular organisms and thus this previously unrecognized proapoptotic signal, masked by concomitant NF-kappa B activation, likely required intracellular infection. Our studies demonstrate that a bacterial organism can exert an antiapoptotic effect, thus modulating the host cell's apoptotic response to its own advantage by potentially allowing the host cell to remain as a site of infection.
Figures


References
-
- Silverman D J, Santucci L A. Ann NY Acad Sci. 1990;590:111–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

