Src interacts with dynamin and synapsin in neuronal cells
- PMID: 9539797
- PMCID: PMC22549
- DOI: 10.1073/pnas.95.8.4673
Src interacts with dynamin and synapsin in neuronal cells
Abstract
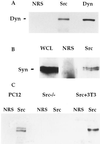
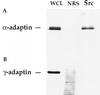
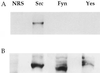
The nonreceptor tyrosine kinase Src is expressed at a high level in cells that are specialized for regulated secretion, such as the neuron, and is concentrated on secretory vesicles or at the site of exocytosis. To investigate the possibility that Src may play a role in regulating membrane traffic, we searched for neuronal proteins that will interact with Src. The SH3 domain of Src, but not that of the splice variant N-Src, bound to three proteins from mouse synaptosomes or PC12 cells: dynamin, synapsin Ia, and synapsin Ib. Dynamin and the synapsins coprecipitated with Src from PC12 cell extracts, and they colocalized with a subset of Src in the PC12 cell by immunofluorescence. Neither dynamin nor the synapsins were phosphorylated by Src, suggesting that the interaction of these proteins serves to direct the kinase activity of Src toward other proteins in the vesicle population. In immunoprecipitates containing Src and dynamin, the clathrin adaptor protein alpha-adaptin was also found. The association of Src and synapsin suggests a role for Src in the life cycle of the synaptic vesicle. The identification of a complex containing Src, dynamin, and alpha-adaptin indicates that Src may play a more general role in membrane traffic as well.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

