Sucrose is a signal molecule in assimilate partitioning
- PMID: 9539816
- PMCID: PMC22568
- DOI: 10.1073/pnas.95.8.4784
Sucrose is a signal molecule in assimilate partitioning
Abstract
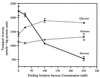
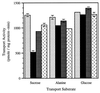
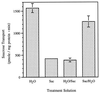
The proton-sucrose symporter mediates the key transport step in the resource distribution system that allows many plants to function as multicellular organisms. In the results reported here, we identify sucrose as a signaling molecule in a previously undescribed signal-transduction pathway that regulates the symporter. Sucrose symporter activity declined in plasma membrane vesicles isolated from leaves fed exogenous sucrose via the xylem transpiration stream. Symporter activity dropped to 35-50% of water controls when the leaves were fed 100 mM sucrose and to 20-25% of controls with 250 mM sucrose. In contrast, alanine symporter and glucose transporter activities did not change in response to sucrose treatments. Decreased sucrose symporter activity was detectable after 8 h and reached a maximum by 24 h. Kinetic analysis of transport activity showed a decrease in Vmax. RNA gel blot analysis revealed a decrease in symporter message levels, suggesting a drop in transcriptional activity or a decrease in mRNA stability. Control experiments showed that these responses were not the result of changing osmotic conditions. Equal molar concentrations of hexoses did not elicit the response, and mannoheptulose, a hexokinase inhibitor, did not block the sucrose effect. These data are consistent with a sucrose-specific response pathway that is not mediated by hexokinase as the sugar sensor. Sucrose-dependent changes in the sucrose symporter were reversible, suggesting this sucrose-sensing pathway can modulate transport activity as a function of changing sucrose concentrations in the leaf. These results demonstrate the existence of a signaling pathway that can control assimilate partitioning at the level of phloem translocation.
Figures


References
-
- Avigad G. In: Encyclopedia of Plant Physiology. Loewus F A, Tanner W, editors. 13A. Berlin: Springer; 1982. pp. 217–347.
-
- Ziegler H, Zimmermann M H. In: Transport in Plants I: Phloem Transport. Zimmermann M H, Milburn J A, editors. Berlin: Springer; 1975. pp. 480–503.
-
- Gifford R M, Thorne J H, Hitz W D, Giaquinta R T. Science. 1984;225:801–808. - PubMed
-
- Giaquinta R T. Annu Rev Plant Physiol. 1983;34:347–387.
-
- Bush D R. Photosynthesis Res. 1992;32:155–165. - PubMed
LinkOut - more resources
Full Text Sources

