Comparison of culture- and non-culture-based methods for quantification of viral load and resistance to antiretroviral drugs in patients given zidovudine monotherapy
- PMID: 9542937
- PMCID: PMC104689
- DOI: 10.1128/JCM.36.4.1056-1063.1998
Comparison of culture- and non-culture-based methods for quantification of viral load and resistance to antiretroviral drugs in patients given zidovudine monotherapy
Abstract
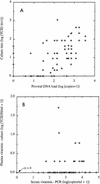
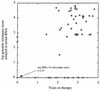
Virological assays for human immunodeficiency virus type 1 load and drug resistance can broadly be divided into culture-based and molecular biology-based methods. Culture-based methods give a direct measure of infectious virus load and phenotypic drug resistance, whereas molecular biology-based methods are indirect, assaying nucleic acid levels to determine virus load and point mutations associated with drug resistance. We have compared culture-based and non-culture-based methods for patients enrolled in a placebo-controlled trial of zidovudine (the Concorde Trial). Virus loads were assayed by culture of peripheral blood mononuclear cells (PBMCs) or quantitative PCR, and drug resistance was assayed in culture or in a quantitative, PCR-based point mutation assay. The rates of detection of viremia and drug resistance were higher by PCR than by culture for this population of subjects. Comparison of the virus loads by the two measures showed a good correlation for virus loads in PBMCs but a poor correlation for virus loads in plasma. The latter result probably reflected the inaccuracies of culture in assaying plasma with the low infectious virus titers seen in the study population. The concordance of phenotypic and genotypic drug resistance methods was high, with all phenotypically resistant isolates having at least one resistance-associated mutation and with no mutations being found in a drug-sensitive isolate. Genomic resistance scores (weighted sums of levels of resistance mutations) showed good correlations with the levels of phenotypic resistance, and both resistance measures were observed to increase as the duration of exposure to drug increased. Overall, non-culture-based methods were shown to correlate well with culture-based methods and offer a low-cost, high-throughput alternative. However, culture-based methods remain the final arbiters of infectious virus load and phenotypic drug resistance and are unlikely to be superseded entirely.
Figures


References
-
- Aoki-Sei S, Yarchoan R, Kageyama S, Hoekzema D T, Pluda J M, Wyvill K M, Broder S, Mitsuya H. Plasma HIV-1 viraemia in HIV-1 infected individuals assessed by polymerase chain reaction. AIDS Res Hum Retroviruses. 1992;8:1263–1270. - PubMed
-
- Bieniasz P D, Ariyoshi K, Bourelly M A D, Bloor S, Foxall R B, Harwood E C, Weber J N. Variable relationship between proviral DNA load and infectious virus titre in the peripheral blood mononuclear cells of HIV-1 infected individuals. AIDS. 1993;7:803–806. - PubMed
-
- Boucher C A B, O’Sullivan E, Mulder J W, Ramaurtarsing C, Kellam P, Darby G, Lange J M A, Goudsmit J, Larder B A. Ordered appearance of zidovudine resistance mutations during treatment of 18 human immunodeficiency virus-positive subjects. J Infect Dis. 1992;165:105–110. - PubMed
-
- Brun-Vezinet F, Ingrand D, Deforges J, Gochi K, Ferchal F, Schmitt M-P, Jung M, Masquelier B, Aubert J, Buffet-Janvresse C, Fleury H. HIV-1 sensitivity to zidovudine: a consensus culture technique validated by genotypic analysis of the reverse transcriptase. J Virol Methods. 1992;37:177–188. - PubMed
-
- Byrnes V W, Sardana V V, Schleif W A, Condra J A, Wolfgang J A, Long W J, Schneider C L. Comprehensive mutant enzyme and viral variant assessment of human immunodeficiency virus type 1 reverse transcriptase resistance to nonnucleoside inhibitors. Antimicrob Agents Chemother. 1993;27:1576–1579. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

