Optimization of specimen-handling procedures for accurate quantitation of levels of human immunodeficiency virus RNA in plasma by reverse transcriptase PCR
- PMID: 9542939
- PMCID: PMC104691
- DOI: 10.1128/JCM.36.4.1070-1073.1998
Optimization of specimen-handling procedures for accurate quantitation of levels of human immunodeficiency virus RNA in plasma by reverse transcriptase PCR
Abstract
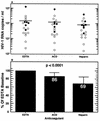
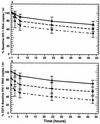
Human immunodeficiency virus type 1 (HIV-1) RNA levels in plasma are currently widely used clinically for prognostication and in monitoring antiretroviral therapy. Accurate and reproducible results are critical for patient management. To determine the effects of specimen collection and handling procedures on quantitative measurement of HIV-1 RNA, we compared anticoagulants and sample processing times. Whole blood was collected from 20 HIV-1-infected patients in EDTA, acid citrate dextrose (ACD), and heparin tubes, aliquoted, and stored at room temperature. Plasma was separated from whole-blood aliquots prepared at < or =1, 3, 6, 24, and 48 h postcollection and then stored at -70 degrees C until use. HIV-1 RNA levels were determined by the AMPLICOR HIV-1 MONITOR assay. Heparinized plasma samples, which were pretreated with heparinase prior to analysis, had the lowest baseline HIV-1 RNA levels. In the first 6 h, HIV-1 RNA levels decreased by 10, 20, and 31% in EDTA, ACD, and heparin tubes, respectively. From 6 to 48 h postcollection, HIV-1 RNA levels decreased in all anticoagulants, albeit at a slower, more consistent rate. Our results indicate that EDTA should be the anticoagulant of choice for plasma HIV-1 RNA measurement by reverse transcriptase PCR, but ACD tubes are acceptable if the plasma is separated within 6 h of blood collection. Caution must be applied in the interpretation of absolute HIV-1 RNA copy number values obtained with suboptimal specimen collection and processing procedures.
Figures
References
-
- Beutler E, Gelbart T, Kuhl W. Interference of heparin with polymerase chain reaction. BioTechniques. 1990;90:166–167. - PubMed
-
- Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morbid Mortal Weekly Rep. 1994;43:1–9.
-
- Centers for Disease Control. Guidelines for prophylaxis against Pneumocystis carinii pneumonia for persons infected with human immunodeficiency virus. Morbid Mortal Weekly Rep. 1989;5:1–9. - PubMed
-
- Dewar R L, Highbarger H C, Sarmiento M D, Todd J A, Vasudevachari M B, Davey R T, Jr, Kovacs J A, Salzman N P, Lane H C, Urdea M S. Application of branched DNA signal amplification to monitor human immunodeficiency virus type 1 burden in human plasma. J Infect Dis. 1994;170:1172–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

