19F nuclear magnetic resonance as a tool to investigate microbial degradation of fluorophenols to fluorocatechols and fluoromuconates
- PMID: 9546160
- PMCID: PMC106138
- DOI: 10.1128/AEM.64.4.1256-1263.1998
19F nuclear magnetic resonance as a tool to investigate microbial degradation of fluorophenols to fluorocatechols and fluoromuconates
Abstract
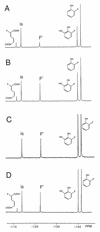
A method was developed to study the biodegradation and oxidative biodehalogenation of fluorinated phenols by 19F nuclear magnetic resonance (NMR). Characterization of the 19F NMR spectra of metabolite profiles of a series of fluorophenols, converted by purified phenol hydroxylase, catechol 1,2-dioxygenase, and/or by the yeast-like fungus Exophiala jeanselmei, provided possibilities for identification of the 19F NMR chemical shift values of fluorinated catechol and muconate metabolites. As an example, the 19F NMR method thus defined was used to characterize the time-dependent metabolite profiles of various halophenols in either cell extracts or in incubations with whole cells of E. jeanselmei. The results obtained for these two systems are similar, except for the level of muconates observed. Altogether, the results of the present study describe a 19F NMR method which provides an efficient tool for elucidating the metabolic pathways for conversion of fluorine-containing phenols by microorganisms, with special emphasis on possibilities for biodehalogenation and detection of the type of fluorocatechols and fluoromuconates involved. In addition, the method provides possibilities for studying metabolic pathways in vivo in whole cells.
Figures

References
-
- Blasco R, Wittich R-M, Mallavarapu M, Timmis K N, Pieper D H. From xenobiotic to antibiotic, formation of protoanemonin from 4-chlorocatechol by enzymes of the 3-oxoadipate pathway. J Biol Chem. 1995;270:29229–29235. - PubMed
-
- Cabanac S, Marlet-Martino M C, Bon M, Martino R, Nedelec J F, Dimicoli J L. Direct 19F NMR spectroscopic observation of 5-fluorouracil metabolism in the isolated perfused mouse liver model. NMR Biomed. 1988;1:113–120. - PubMed
-
- Cass A E G, Ribbons D W, Rossiter J T, Williams S R. Biotransformation of aromatic compounds. Monitoring fluorinated analogues by NMR. FEBS Lett. 1987;220:353–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

