G-Protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor
- PMID: 9547222
- PMCID: PMC6792650
- DOI: 10.1523/JNEUROSCI.18-09-03138.1998
G-Protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor
Abstract
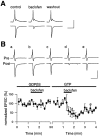
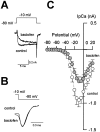
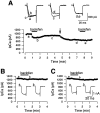
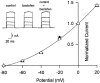
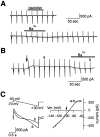
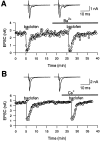
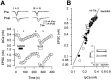
Presynaptic GABAB receptors play a regulatory role in central synaptic transmission. To elucidate their underlying mechanism of action, we have made whole-cell recordings of calcium and potassium currents from a giant presynaptic terminal, the calyx of Held, and EPSCs from its postsynaptic target in the medial nucleus of the trapezoid body of rat brainstem slices. The GABAB receptor agonist baclofen suppressed EPSCs and presynaptic calcium currents but had no effect on voltage-dependent potassium currents. The calcium current-EPSC relationship measured during baclofen application was similar to that observed on reducing [Ca2+]o, suggesting that the presynaptic inhibition generated by baclofen is caused largely by the suppression of presynaptic calcium influx. Presynaptic loading of the GDP analog guanosine-5'-O-(2-thiodiphosphate) (GDPbetaS) abolished the effect of baclofen on both presynaptic calcium currents and EPSCs. The nonhydrolyzable GTP analog guanosine 5'-O-(3-thiotriphosphate) (GTPgammaS) suppressed presynaptic calcium currents and occluded the effect of baclofen on presynaptic calcium currents and EPSCs. Photoactivation of GTPgammaS induced an inward rectifying potassium current at the calyx of Held, whereas baclofen had no such effect. We conclude that presynaptic GABAB receptors suppress transmitter release through G-protein-coupled inhibition of calcium currents.
Figures

References
-
- Andrade R, Malenka RC, Nicoll RA. A G-protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
