Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer's fibrils
- PMID: 9547230
- PMCID: PMC6792661
- DOI: 10.1523/JNEUROSCI.18-09-03213.1998
Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer's fibrils
Abstract
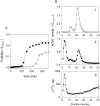
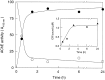
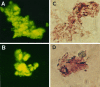
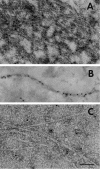
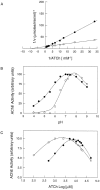
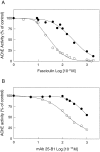
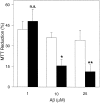
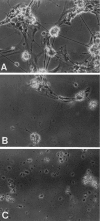
Brain acetylcholinesterase (AChE) forms stable complexes with amyloid-beta peptide (Abeta) during its assembly into filaments, in agreement with its colocalization with the Abeta deposits of Alzheimer's brain. The association of the enzyme with nascent Abeta aggregates occurs as early as after 30 min of incubation. Analysis of the catalytic activity of the AChE incorporated into these complexes shows an anomalous behavior reminiscent of the AChE associated with senile plaques, which includes a resistance to low pH, high substrate concentrations, and lower sensitivity to AChE inhibitors. Furthermore, the toxicity of the AChE-amyloid complexes is higher than that of the Abeta aggregates alone. Thus, in addition to its possible role as a heterogeneous nucleator during amyloid formation, AChE, by forming such stable complexes, may increase the neurotoxicity of Abeta fibrils and thus may determine the selective neuronal loss observed in Alzheimer's brain.
Figures
References
-
- Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor α1-antichymotrypsin in the brain amyloid deposits of the Alzheimer’s disease. Cell. 1988;52:487–501. - PubMed
-
- Alvarez A, Bronfman F, Pérez CA, Vicente M, Garrido J, Inestrosa NC. Acetylcholinesterase, a senile plaque component, affects the fibrillogenesis of amyloid-β-peptides. Neurosci Lett. 1995;201:49–52. - PubMed
-
- Alvarez A, Opazo C, Alarcon R, Garrido J, Inestrosa NC. Acetylcholinesterase promotes the aggregation of amyloid-β-peptide fragments by forming a complex with the growing fibrils. J Mol Biol. 1997;272:348–361. - PubMed
-
- Barcikowska M, Wisniewski HM, Bancher C, Grundke-Iqbal I. About the presence of paired helical filaments in dystrophic neurites participating in the plaque formation. Acta Neuropathol (Berl) 1989;78:225–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
