Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression
- PMID: 9547239
- PMCID: PMC6792660
- DOI: 10.1523/JNEUROSCI.18-09-03314.1998
Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression
Abstract
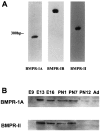
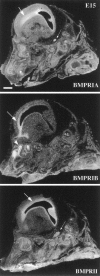
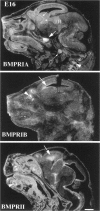
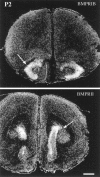
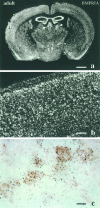
Characterization of bone morphogenetic protein receptor (BMPR) expression during development is necessary for understanding the role of these factors during neural maturation. In this study, in situ hybridization analyses demonstrate that BMP-specific type I (BMPR-IA and BMPR-IB) and type II (BMPR-II) receptor mRNAs are expressed at significant levels in multiple regions of the CNS, cranial ganglia, and peripheral sensory and autonomic ganglia during the embryonic and neonatal periods. All three BMP receptor subunits are expressed within periventricular generative zones. BMPR-IA is more abundant than the other receptor subtypes, with widespread expression in the brain, cranial ganglia, and peripheral ganglia. By contrast, BMPR-IB mRNA displays significant expression within more restricted regions, including the anterior olfactory nuclei. BMPR-II mRNA exhibits peak expression within the cerebellar Purkinje cell layer and the hippocampus, as well as within cranial ganglia. The distribution of BMP receptors within large neurons in adult dorsal root ganglia suggested a possible role in regulating expression of the neurotrophin receptor trkC. This hypothesis was tested in explant cultures of embryonic day 15 (E15) and postnatal day 1 (P1) sympathetic superior cervical ganglia (SCG). Treatment of the E15 or the P1 SCG with BMP-2 induced expression of trkC mRNA and responsiveness of sympathetic neurons to NT3 as measured by neurite outgrowth. The pattern of expression of BMP receptors in embryonic brain suggests several potentially novel areas for further developmental analysis and supports numerous recent studies that indicate that BMPs have a broad range of cellular functions during neural development and in adult life.
Figures






References
-
- Arkell R, Beddington RSP. BMP-7 influences pattern and growth of the developing hindbrain of mouse embryos. Development. 1997;124:1–12. - PubMed
-
- Birren SJ, Anderson DJ. A V-myc-immortalized sympathoadrenal progenitor cell line in which neuronal differentiation is initiated by FGF but not NGF. Neuron. 1990;4:189–201. - PubMed
-
- Coughlin MD, Collins MB (1985) Nerve growth factor-independent development of embryonic mouse sympathetic neurons in dissociated cell culture Dev Biol 110:392–401. - PubMed
-
- D’Alessandro JS, Vetz-Aldape J, Wang EA. Bone morphogenetic proteins induce differentiation in astrocyte lineage cells. Growth Factors. 1994;11:53–69. - PubMed
-
- Dale L, Howes G, Price BM, Smith JC. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992;115:573–585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
