Development of the mouse inner ear and origin of its sensory organs
- PMID: 9547240
- PMCID: PMC6792659
- DOI: 10.1523/JNEUROSCI.18-09-03327.1998
Development of the mouse inner ear and origin of its sensory organs
Abstract
The molecular mechanisms dictating the morphogenesis and differentiation of the mammalian inner ear are largely unknown. To better elucidate the normal development of this organ, two approaches were taken. First, the membranous labyrinths of mouse inner ears ranging from 10.25 to 17 d postcoitum (dpc) were filled with paint to reveal their gross development. Particular attention was focused on the developing utricle, saccule, and cochlea. Second, we used bone morphogenetic protein 4 (BMP4) and lunatic fringe (Fng) as molecular markers to identify the origin of the sensory structures. Our data showed that BMP4 was an early marker for the superior, lateral, and posterior cristae, whereas Fng served as an early marker for the macula utriculi, macula sacculi, and the sensory portion of the cochlea. The posterior crista was the first organ to appear at 11.5 dpc and was followed by the superior crista, the lateral crista, and the macula utriculi at 12 dpc. The macula sacculi and the cochlea were present at 12 dpc but became distinguishable from each other by 13 dpc. Based on the gene expression patterns, the anterior and lateral cristae may share a common origin. Similarly, three sensory organs, the macula utriculi, macula sacculi, and cochlea, seem to arise from a single region of the otocyst.
Figures


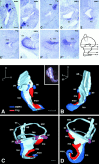
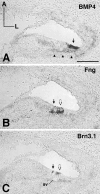
References
-
- Bast TH, Anson BJ. The temporal bone and the ear, pp 30–105. Thomas; Springfield, IL: 1949. The otic labyrinth.
-
- Bissonnette JP, Fekete DM. Standard atlas of the gross anatomy of the developing inner ear of the chicken. J Comp Neurol. 1996;174:1–11. - PubMed
-
- Cohen B, Bashirullah A, Dagnino L, Campbell C, Fisher WW, Leow CC, Whiting E, Ryan D, Zinyk D, Boulianne G, Hui CC, Gallie B, Phillips RA, Lipshitz HD, Egan SE. Fringe boundaries coincide with Notch-dependent patterning centres in mammals and alter Notch-dependent development in Drosophila. Nat Genet. 1997;16:283–288. - PubMed
-
- Cohen GM, Cotanche DA. Development of the sensory receptors and their innervation in the chick cochlea. In: Romand AL, editor. Development of auditory and vestibular systems, Vol 2. Elsevier; New York: 1992. pp. 101–138.
-
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
