Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina
- PMID: 9547245
- PMCID: PMC6792640
- DOI: 10.1523/JNEUROSCI.18-09-03373.1998
Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina
Abstract
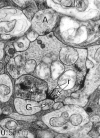
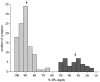
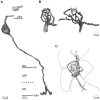
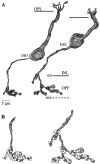
Perception of hue is opponent, involving the antagonistic comparison of signals from different cone types. For blue versus yellow opponency, the antagonism is first evident at a ganglion cell with firing that increases to stimulation of short wavelength-sensitive (S) cones and decreases to stimulation of middle wavelength-sensitive (M) and long wavelength-sensitive (L) cones. This ganglion cell, termed blue-yellow (B-Y), has a distinctive morphology with dendrites in both ON and OFF strata of the inner plexiform layer (Dacey and Lee, 1994). Here we report the synaptic circuitry of the cell and its spatial density. Reconstructing neurons in macaque fovea from electron micrographs of serial sections, we identified six ganglion cells that branch in both strata and have similar circuitry. In the ON stratum each cell collects approximately 33 synapses from bipolar cells traced back exclusively to invaginating contacts from S cones, and in the OFF stratum each cell collects approximately 14 synapses from bipolar cells (types DB2 and DB3) traced to basal synapses from approximately 20 M and L cones. This circuitry predicts that spatially coincident blue-yellow opponency arises at the level of the cone output via expression of different glutamate receptors. S cone stimuli suppress glutamate release onto metabotropic receptors of the S cone bipolar cell dendrite, thereby opening cation channels, whereas M and L cone stimuli suppress glutamate release onto ionotropic glutamate receptors of DB2 and DB3 cell dendrites, thereby closing cation channels. Although the B-Y cell is relatively rare (3% of foveal ganglion cells), its spatial density equals that of the S cone; thus it could support psychophysical discrimination of a blue-yellow grating down to the spatial cutoff of the S cone mosaic.
Figures





References
-
- Atick JJ. Could information theory provide an ecological theory of sensory processing? Network. 1992;3:213–251. - PubMed
-
- Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Philos Trans R Soc Lond B Biol Sci. 1969;255:109–184. - PubMed
-
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088. - PubMed
-
- Buchsbaum G, Gottschalk A. Trichromacy, opponent colours coding and optimum colour information transmission in the retina. Proc R Soc Lond B Biol Sci. 1983;220:89–113. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
