GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus
- PMID: 9547246
- PMCID: PMC6792657
- DOI: 10.1523/JNEUROSCI.18-09-03386.1998
GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus
Abstract
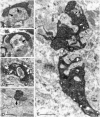
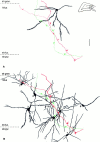
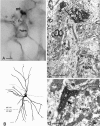
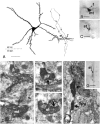
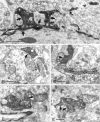
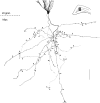
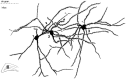
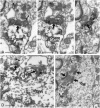
Dentate granule cells communicate with their postsynaptic targets by three distinct terminal types. These include the large mossy terminals, filopodial extensions of the mossy terminals, and smaller en passant synaptic varicosities. We examined the postsynaptic targets of mossy fibers by combining in vivo intracellular labeling of granule cells, immunocytochemistry, and electron microscopy. Single granule cells formed large, complex "mossy" synapses on 11-15 CA3 pyramidal cells and 7-12 hilar mossy cells. In contrast, GABAergic interneurons, identified with immunostaining for substance P-receptor, parvalbumin, and mGluR1a-receptor, were selectively innervated by very thin (filopodial) extensions of the mossy terminals and by small en passant boutons in both the hilar and CA3 regions. These terminals formed single, often perforated, asymmetric synapses on the cell bodies, dendrites, and spines of GABAergic interneurons. The number of filopodial extensions and small terminals was 10 times larger than the number of mossy terminals. These findings show that in contrast to cortical pyramidal neurons, (1) granule cells developed distinct types of terminals to affect interneurons and pyramidal cells and (2) they innervated more inhibitory than excitatory cells. These findings may explain the physiological observations that increased activity of granule cells suppresses the overall excitability of the CA3 recurrent system and may form the structural basis of the target-dependent regulation of glutamate release in the mossy fiber system.
Figures




References
-
- Acsády L, Katona I, Gulyás AI, Shigemoto R, Freund TF. Immunostaining for substance P receptor labels GABAergic cells with distinct termination patterns in the hippocampus. J Comp Neurol. 1997;378:320–336. - PubMed
-
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. - PubMed
-
- Amaral DG. Synaptic extensions from the mossy fibers of the fascia dentata. Anat Embryol. 1979;155:241–251. - PubMed
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
