T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching
- PMID: 9547331
- PMCID: PMC2212236
- DOI: 10.1084/jem.187.8.1193
T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching
Abstract
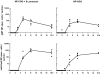
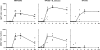
The respective production of specific immunoglobulin (Ig)G2a or IgG1 within 5 d of primary immunization with Swiss type mouse mammary tumor virus [MMTV(SW)] or haptenated protein provides a model for the development of T helper 1 (Th1) and Th2 responses. The antibody-producing cells arise from cognate T cell B cell interaction, revealed by the respective induction of Cgamma2a and Cgamma1 switch transcript production, on the third day after immunization. T cell proliferation and upregulation of mRNA for interferon gamma in response to MMTV(SW) and interleukin 4 in response to haptenated protein also starts during this day. It follows that there is minimal delay in these responses between T cell priming and the onset of cognate interaction between T and B cells leading to class switching and exponential growth. The Th1 or Th2 profile is at least partially established at the time of the first cognate T cell interaction with B cells in the T zone. The addition of killed Bordetella pertussis to the hapten-protein induces nonhapten-specific IgG2a and IgG1 plasma cells, whereas the anti-hapten response continues to be IgG1 dominated. This indicates that a Th2 response to hapten-protein can proceed in a node where there is substantial Th1 activity.
Figures

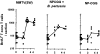



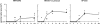
References
-
- Kelso A. Th1 and Th2 subsets: paradigms lost? . Immunol Today. 1995;16:374–379. - PubMed
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
-
- Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. - PubMed
-
- Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. - PubMed
-
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. 2 types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

