T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells
- PMID: 9547334
- PMCID: PMC2212228
- DOI: 10.1084/jem.187.8.1225
T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells
Abstract
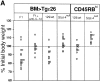
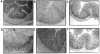
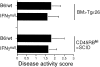
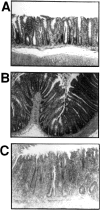
The requirements for interleukin (IL)-12/signal transducer and activator of transcription (Stat)-4 signaling and induction of T cell-specific interferon (IFN)-gamma expression in the development of T helper cell (Th)1-type pathology were examined in two different models of experimental colitis. In each model, abnormal reconstitution of the T cell compartment in immunodeficient mice by adoptive cell transfer leads to a wasting syndrome and inflammation of the colon, induced by IFN-gamma and tumor necrosis factor (TNF)-alpha-producing T cells. We show here that treatment with anti-IL-12 antibodies in one of the models, or reconstitution with T cells from Stat-4-deficient (Stat-4(null)) mice in both models resulted in a milder disease in the majority of recipient animals, compared with those that were left untreated or that had been reconstituted with wt cells. Protected mice in each group also harbored lower frequencies of IFN-gamma-producing T cells than did diseased mice, suggesting that effects on wasting and colitis resulted from the attenuation of IFN-gamma expression by T cells. To test whether the development of pathogenic T cells in the two colitis models was directly dependent on T cell-specific IFN-gamma expression, IFN-gammanull donors were used for T cell reconstitution in each system. Surprisingly, large numbers of IFN-gammanull-reconstituted mice developed wasting and colitis, which in many cases was of comparable severity to that seen in animals reconstituted with wt cells. Furthermore, T cells from these animals expressed TNF-alpha, demonstrating that they had retained the ability to produce another proinflammatory cytokine. Taken together, these results demonstrate that in some forms of chronic experimental colitis the development of pathogenic T cells is influenced predominantly, though not exclusively, by IL-12 via the actions of Stat-4 proteins. Furthermore, our data suggest that in the models of colitis studied here the effects of IL-12/Stat-4 or other Th1 promoting pathways are not limited to the induction of IFN-gamma gene expression in T lymphocytes.
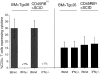
Figures





References
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325:928–937. - PubMed
-
- MacDonald TT. Gastrointestinal inflammation. Inflammatory bowel disease in knockout mice. Curr Biol. 1994;3:261–263. - PubMed
-
- Sartor RB. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995;24:475–507. - PubMed
-
- Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–539. - PubMed
-
- Simpson, S.J., G.A. Hollander, E. Mizoguchi, A.K. Bhan, B. Wang, and C. Terhorst. 1996. Defects in T-cell regulation: lessons for inflammatory bowel disease. In Essentials of Mucosal Immunology. M.F. Kagnoff and H. Kiyono, editors. Academic Press Inc., San Diego, CA. 291–304.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

