Tolerization of anti-Galalpha1-3Gal natural antibody-forming B cells by induction of mixed chimerism
- PMID: 9547344
- PMCID: PMC2212239
- DOI: 10.1084/jem.187.8.1335
Tolerization of anti-Galalpha1-3Gal natural antibody-forming B cells by induction of mixed chimerism
Abstract
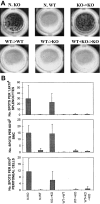
Xenotransplantation could overcome the severe shortage of allogeneic organs, a major factor limiting organ transplantation. Unfortunately, transplantation of organs from pigs, the most suitable potential donor species, results in hyperacute rejection in primate recipients, due to the presence of anti-Galalpha1-3Gal (Gal) natural antibodies (NAbs) in their sera. We evaluated the ability to tolerize anti-Gal NAb-producing B cells in alpha1,3-galactosyltransferase knockout (GalT KO) mice using bone marrow transplantation (BMT) from GalT+/+ wild-type (WT) mice. Lasting mixed chimerism was achieved in KO mice by cotransplantation of GalT KO and WT marrow after lethal irradiation. The levels of anti-Gal NAb in sera of mixed chimeras were reduced markedly 2 wk after BMT, and became undetectable at later time points. Immunization with Gal+/+ xenogeneic cells failed to stimulate anti-Gal antibody production in mixed chimeras, whereas the production of non-Gal-specific antixenoantigen antibodies was stimulated. An absence of anti-Gal-producing B cells was demonstrated by enzyme-linked immunospot assays in mixed KO + WT --> KO chimeras. Thus, mixed chimerism efficiently induces anti-Gal-specific B cell tolerance in addition to T cell tolerance, providing a single approach to overcoming both the humoral and the cellular immune barriers to discordant xenotransplantation.
Figures




References
-
- Cooper DKC. Xenografting: how great is the clinical need? . Xeno. 1993;2:25–26.
-
- Platt JL. Xenotransplantation: recent progress and current perspectives. Curr Opin Immunol. 1996;8:721–728. - PubMed
-
- Sachs DH. The pig as a xenograft donor. Pathol Biol. 1994;42:217–219. - PubMed
-
- Cooper, D.K.C., Y. Ye, L.L. Rolf, Jr., and N. Zuhdi. 1991. The pig as potential organ donor for man. In Xenotransplantation. D.K.C. Cooper, E. Kemp, K. Reemtsma, and D.J.G. White, editors. Springer-Verlag, Heidelberg. 481–500.
-
- Auchincloss HJ. Xenogeneic transplantation. A review. Transplantation (Baltimore) 1988;46:1–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

