Peroxisome biogenesis: involvement of ARF and coatomer
- PMID: 9548716
- PMCID: PMC2148451
- DOI: 10.1083/jcb.141.2.373
Peroxisome biogenesis: involvement of ARF and coatomer
Abstract
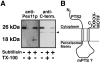
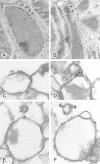
Peroxisomal membrane protein (Pmp)26p (RnPex11p), a major constituent of induced rat liver peroxisomal membrane, was found to contain a COOH-terminal, cytoplasmically exposed consensus dilysine motif with the potential to bind coatomer. Biochemical as well as immunocytochemical evidence is presented showing that peroxisomes incubated with preparations of bovine brain or rat liver cytosol recruit ADP-ribosylation factor (ARF) and coatomer in a strictly guanosine 5'-O-(3-thiotriphosphate)-dependent manner. Consistent with this observation, ldlF cells expressing a temperature-sensitive mutant version of the epsilon-subunit of coatomer exhibit elongated tubular peroxisomes possibly due to impaired vesiculation at the nonpermissive temperature. Since overexpression of Pex11p in Chinese hamster ovary wild-type cells causes proliferation of peroxisomes, these data suggest that Pex11p plays an important role in peroxisome biogenesis by supporting ARF- and coatomer-dependent vesiculation of the organelles.
Figures









References
-
- Antoine B, Levrat F, Vallet V, Berbar T, Cartier N, Dubois N, Briand P, Kahn A. Gene expression in hepatocyte-like lines established by targeted carcinogenesis in transgenic mice. Exp Cell Res. 1992;200:175–185. - PubMed
-
- Baerends RJ, Rasmussen SW, Hilbrands RE, van der Heide M, Faber KN, Reuvekamp PT, Kiel JA, Cregg JM, van der Klei IJ, Veenhuis M. The Hansenula polymorphaPER9 gene encodes a peroxisomal membrane protein essential for peroxisome assembly and integrity. J Biol Chem. 1996;271:8887–8894. - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–908. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

