Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions
- PMID: 9548718
- PMCID: PMC2148457
- DOI: 10.1083/jcb.141.2.397
Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions
Abstract
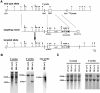
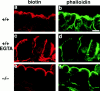
Occludin is the only known integral membrane protein of tight junctions (TJs), and is now believed to be directly involved in the barrier and fence functions of TJs. Occludin-deficient embryonic stem (ES) cells were generated by targeted disruption of both alleles of the occludin gene. When these cells were subjected to suspension culture, they aggregated to form simple, and then cystic embryoid bodies (EBs) with the same time course as EB formation from wild-type ES cells. Immunofluorescence microscopy and ultrathin section electron microscopy revealed that polarized epithelial (visceral endoderm-like) cells were differentiated to delineate EBs not only from wild-type but also from occludin-deficient ES cells. Freeze fracture analyses indicated no significant differences in number or morphology of TJ strands between wild-type and occludin-deficient epithelial cells. Furthermore, zonula occludens (ZO)-1, a TJ-associated peripheral membrane protein, was still exclusively concentrated at TJ in occludin-deficient epithelial cells. In good agreement with these morphological observations, TJ in occludin-deficient epithelial cells functioned as a primary barrier to the diffusion of a low molecular mass tracer through the paracellular pathway. These findings indicate that there are as yet unidentified TJ integral membrane protein(s) which can form strand structures, recruit ZO-1, and function as a barrier without occludin.
Figures









References
-
- Anderson JM, Fanning AS, Lapierre L, Van Itallie CM. Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction. Biochem Soc Trans. 1995;23:470–475. - PubMed
-
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

