Knockout and knockin of the beta1 exon D define distinct roles for integrin splice variants in heart function and embryonic development
- PMID: 9553049
- PMCID: PMC316718
- DOI: 10.1101/gad.12.8.1202
Knockout and knockin of the beta1 exon D define distinct roles for integrin splice variants in heart function and embryonic development
Abstract
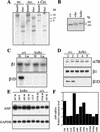
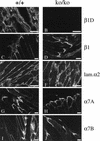
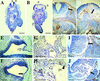
The beta1D integrin is a recently characterized isoform of the beta1 subunit that is specifically expressed in heart and skeletal muscle. In this study we have assessed the function of the beta1D integrin splice variant in mice by generating, for the first time, Cre-mediated exon-specific knockout and knockin strains for this splice variant. We show that removal of the exon for beta1D leads to a mildly disturbed heart phenotype, whereas replacement of beta1A by beta1D results in embryonic lethality with a plethora of developmental defects, in part caused by the abnormal migration of neuroepithelial cells. Our data demonstrate that the splice variants A and D are not functionally equivalent. We propose that beta1D is less efficient than beta1A in mediating the signaling that regulates cell motility and responses of the cells to mechanical stress.
Figures






References
-
- Almeida EAC, Huovila APJ, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG, White JM. Mouse egg integrin α6β1 functions as a sperm receptor. Cell. 1995;81:1095–1104. - PubMed
-
- Altruda F, Cervella P, Tarone G, Botta C, Balzac F, Stefanuto G, Silengo L. A human integrin β1 subunit with a unique cytoplasmic domain generated by alternative mRNA processing. Gene. 1990;95:261–266. - PubMed
-
- Aumailley M, Mann K, von der Mark H, Timpl R. Cell attachment properties of collagen type IV and Arg-Gly-Asp dependent binding to its α2(VI) and α3(VI) chains. Exp Cell Res. 1989;181:463–474. - PubMed
-
- Baudoin C, Van der Flier A, Borradori L, Sonnenberg A. Genomic organization of the mouse β1 gene: Conservation of the β1D but not of the β1B and β1C integrin splice variants. Cell Adhes Commun. 1996;4:1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
