Suppressor mutation analysis of the sensory rhodopsin I-transducer complex: insights into the color-sensing mechanism
- PMID: 9555883
- PMCID: PMC107127
- DOI: 10.1128/JB.180.8.2033-2042.1998
Suppressor mutation analysis of the sensory rhodopsin I-transducer complex: insights into the color-sensing mechanism
Abstract
The molecular complex containing the phototaxis receptor sensory rhodopsin I (SRI) and transducer protein HtrI (halobacterial transducer for SRI) mediates color-sensitive phototaxis responses in the archaeon Halobacterium salinarum. One-photon excitation of the complex by orange light elicits attractant responses, while two-photon excitation (orange followed by near-UV light) elicits repellent responses in swimming cells. Several mutations in SRI and HtrI cause an unusual mutant phenotype, called orange-light-inverted signaling, in which the cell produces a repellent response to normally attractant light. We applied a selection procedure for intragenic and extragenic suppressors of orange-light-inverted mutants and identified 15 distinct second-site mutations that restore the attractant response. Two of the 3 suppressor mutations in SRI are positioned at the cytoplasmic ends of helices F and G, and 12 suppressor mutations in HtrI cluster at the cytoplasmic end of the second HtrI transmembrane helix (TM2). Nearly all suppressors invert the normally repellent response to two-photon stimulation to an attractant response when they are expressed with their suppressible mutant alleles or in an otherwise wild-type strain. The results lead to a model for control of flagellar reversal by the SRI-HtrI complex. The model invokes an equilibrium between the A (reversal-inhibiting) and R (reversal-stimulating) conformers of the signaling complex. Attractant light and repellent light shift the equilibrium toward the A and R conformers, respectively, and mutations are proposed to cause intrinsic shifts in the equilibrium in the dark form of the complex. Differences in the strength of the two-photon signal inversion and in the allele specificity of suppression are correlated, and this correlation can be explained in terms of different values of the equilibrium constant (Keq) for the conformational transition in different mutants and mutant-suppressor pairs.
Figures
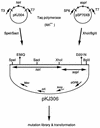

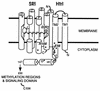

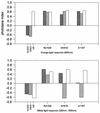



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

