Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli
- PMID: 9555887
- PMCID: PMC107131
- DOI: 10.1128/JB.180.8.2063-2071.1998
Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli
Abstract
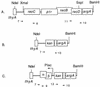
Replacement of Escherichia coli's RecBCD function with phage lambda's Red function generates a strain whose chromosome recombines with short linear DNA fragments at a greatly elevated rate. The rate is at least 70-fold higher than that exhibited by a recBC sbcBC or recD strain. The value of the system is highlighted by gene replacement with a PCR-generated DNA fragment. The deltarecBCD::Plac-red kan replacement allele can be P1 transduced to other E. coli strains, making the hyper-Rec phenotype easily transferable.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

