A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase
- PMID: 9555904
- PMCID: PMC107148
- DOI: 10.1128/JB.180.8.2194-2200.1998
A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase
Abstract
Bacillus subtilis grown at 37 degrees C synthesizes saturated fatty acids with only traces of unsaturated fatty acids (UFAs). However, when cultures growing at 37 degrees C are transferred to 20 degrees C, UFA synthesis is induced. We report the identification and characterization of the gene encoding the fatty acid desaturase of B. subtilis. This gene, called des, was isolated by complementation of Escherichia coli strains with mutations in either of two different genes of UFA synthesis. The des gene encodes a polypeptide of 352 amino acid residues containing the three conserved histidine cluster motifs and two putative membrane-spanning domains characteristic of the membrane-bound desaturases of plants and cyanobacteria. Expression of the des gene in E. coli resulted in desaturation of palmitic acid moieties of the membrane phospholipids to give the novel mono-UFA cis-5-hexadecenoic acid, indicating that the B. subtilis des gene product is a delta5 acyl-lipid desaturase. The des gene was disrupted, and the resulting null mutant strains were unable to synthesize UFAs upon a shift to low growth temperatures. The des null mutant strain grew as well as its congenic parent at 20 or 37 degrees C but showed severely reduced survival during stationary phase. Analysis of operon fusions in which the des promoter directed the synthesis of a lacZ reporter gene showed that des expression is repressed at 37 degrees C, but a shift of cultures from 37 to 20 degrees C resulted in a 10- to 15-fold increase in transcription. This is the first report of a membrane phospholipid desaturase in a nonphotosynthetic organism and the first direct evidence for cold induction of a desaturase.
Figures


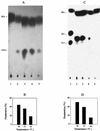


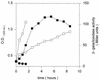
References
-
- Bloch K. β-Hydroxydecanoyl thioester dehydrase. In: Boyer P D, editor. The enzymes. New York, N.Y: Academic Press, Inc.; 1971. pp. 441–464.
-
- Bron S. Plamids. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 75–138.
-
- Cronan J E, Rock C O. Biosynthesis of membrane lipids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 612–636.
-
- de Mendoza D, Grau R, Cronan J E., Jr . Biosynthesis and function of membrane lipids. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 411–421.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

