Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart
- PMID: 9556565
- PMCID: PMC3522116
- DOI: 10.1074/jbc.273.18.10893
Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart
Abstract
The metabolism of 4-hydroxy-trans-2-nonenal (HNE), an alpha, beta-unsaturated aldehyde generated during lipid peroxidation, was studied in isolated perfused rat hearts. High performance liquid chromatography separation of radioactive metabolites recovered from [3H]HNE-treated hearts revealed four major peaks. Based on the retention times of synthesized standards, peak I, which accounted for 20% radioactivity administered to the heart, was identified to be due to glutathione conjugates of HNE. Peaks II and III, containing 2 and 37% radioactivity, were assigned to 1, 4-dihydroxy-2-nonene (DHN) and 4-hydroxy-2-nonenoic acid, respectively. Peak IV was due to unmetabolized HNE. The electrospray ionization mass spectrum of peak I revealed two prominent metabolites with m/z values corresponding to [M + H]+ of HNE and DHN conjugates with glutathione. The presence of 4-hydroxy-2-nonenoic acid in peak III was substantiated using gas chromatography-chemical ionization mass spectroscopy. When exposed to sorbinil, an inhibitor of aldose reductase, no GS-DHN was recovered in the coronary effluent, and treatment with cyanamide, an inhibitor of aldehyde dehydrogenase, attenuated 4-hydroxy-2-nonenoic acid formation. These results show that the major metabolic transformations of HNE in rat heart involve conjugation with glutathione and oxidation to 4-hydroxy-2-nonenoic acid. Further metabolism of the GS-HNE conjugate involves aldose reductase-mediated reduction, a reaction catalyzed in vitro by homogenous cardiac aldose reductase.
Figures

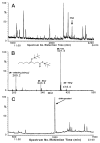
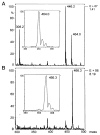



References
-
- Halliwell B, Gutteridge JMC. Methods Enzymol. 1990;186:1–85. - PubMed
-
- Lander HM. FASEB J. 1997;11:118–124. - PubMed
-
- Landers HM, Ogiste JS, Teng KK, Novogrodsky A. J Biol Chem. 1995;270:21195–21198. - PubMed
-
- Sundaresan M, Yu Z-X, Ferrans VJ, Irani K, Finkel T. Science. 1995;270:296–299. - PubMed
-
- Esterbauer H, Schaur RJ, Zollner H. Free Radic Biol Med. 1991;11:81–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

