Elongation of the cytoplasmic tail interferes with the fusion activity of influenza virus hemagglutinin
- PMID: 9557635
- PMCID: PMC109575
- DOI: 10.1128/JVI.72.5.3554-3559.1998
Elongation of the cytoplasmic tail interferes with the fusion activity of influenza virus hemagglutinin
Abstract
The hemagglutinin (HA) of fowl plague virus was lengthened and shortened by site-specific mutagenesis at the cytoplasmic tail, and the effects of these modifications on HA functions were analyzed after expression from a simian virus 40 vector. Elongation of the tail by the addition of one to six histidine (His) residues did not interfere with intracellular transport, glycosylation, proteolytic cleavage, acylation, cell surface expression, and hemadsorption. However, the ability to induce syncytia at a low pH decreased dramatically depending on the number of His residues added. Partial fusion (hemifusion), assayed by fluorescence transfer from octadecylrhodamine-labeled erythrocyte membranes, was also reduced, but even with the mutant carrying six His residues, significant transfer was observed. However, when the formation of fusion pores was examined with hydrophilic fluorescent calcein, transfer from erythrocytes to HA-expressing cells was not observed with the mutant carrying six histidine residues. The addition of different amino acids to the cytoplasmic tail of HA caused an inhibitory effect similar to that caused by the addition of His. On the other hand, a mutant lacking the cytoplasmic tail was still able to fuse at a reduced level. These results demonstrate that elongation of the cytoplasmic tail interferes with the formation and enlargement of fusion pores. Thus, the length of the cytoplasmic tail plays a critical role in the fusion process.
Figures


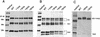

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

