Identification of regions of poliovirus 2BC protein that are involved in cytotoxicity
- PMID: 9557636
- PMCID: PMC109576
- DOI: 10.1128/JVI.72.5.3560-3570.1998
Identification of regions of poliovirus 2BC protein that are involved in cytotoxicity
Abstract
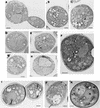
The expression of poliovirus 2BC protein in yeast and mammalian cells leads to a number of metabolic and morphological alterations, such as growth inhibition, intracellular membrane proliferation, blockade of the exocytic pathway, and enhanced membrane permeability. Yeast cells that express poliovirus 2BC in an inducible manner were used to identify the regions of 2BC implicated in the modifications of these cellular functions. Several 2BC deletion mutants were generated to define the minimal portion of 2BC required to alter these activities. Additional deletion mutants that were obtained by random mutagenesis followed by selection in yeast cells provided new insights into the structure and mechanism of action of 2BC. The activity responsible for membrane proliferation is located in 2C, while the activities responsible for membrane permeabilization and inhibition of the exocytic pathway are located in 2B. Several regions of 2B and 2C required for the different functions of 2BC were identified. Thus, the integrity of the N termini of both 2B and 2C is necessary for 2BC-induced cytotoxicity. It is also possible to separate the different cellular alterations provoked by 2BC by the use of several 2BC variants. Deletion of amino acids 52 to 65 in 2B generates a 2BC deletion variant, 2bC deltaAvrII, that still blocks yeast growth but is unable to enhance membrane permeability or to inhibit the exocytic pathway. On the other hand, 2Bcl28*.32b and 2Bcl28*.3c, which contain only 73 and 77 amino acids of 2B, interfere with yeast division and enhance membrane permeability but affect the exocytic pathway only weakly and do not induce membrane proliferation. Our findings indicate that Saccharomyces cerevisiae represents a useful model system to analyze the functions of poliovirus 2BC and show the feasibility of separating the activities assigned to this protein.
Figures



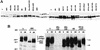

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

