Scaffold attachment region-mediated enhancement of retroviral vector expression in primary T cells
- PMID: 9557654
- PMCID: PMC109594
- DOI: 10.1128/JVI.72.5.3720-3728.1998
Scaffold attachment region-mediated enhancement of retroviral vector expression in primary T cells
Abstract
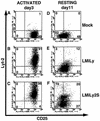
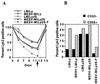
We have studied retroviral transgene expression in primary human lymphocytes. Our data demonstrate that transgene expression is high in activated primary CD4+ T cells but significantly decreased in mitotically quiescent cells. Incorporation of a DNA fragment from the scaffold attachment region (SAR) of the human beta interferon gene into the vector improved transgene expression, particularly in quiescent cells. The SAR element functioned in an orientation-dependent manner and enhanced expression of Moloney murine leukemia virus- and murine embryonic stem cell-based vectors. Clonal analysis of transduced T cells showed that the SAR sequence did not confer position-independent expression on a transgene but rather prevented the decrease of expression when cells became quiescent. The SAR sequence also enhanced transgene expression in T cells generated from retrovirally transduced CD34-enriched hematopoietic progenitor-stem cells in a SCID-hu thymus-liver mouse model. We have used the SAR-containing retroviral vector to express the RevM10 gene, a trans-dominant mutant of the human immunodeficiency virus type 1 (HIV-1) Rev gene. Compared to a standard retroviral vector, the SAR-containing vector was up to 2 orders of magnitude more efficient in inhibiting replication of the HIV-1 virus in infected CD4+ peripheral blood lymphocyte populations in vitro. This is the first demonstration that SAR elements can be used to improve retroviral vector expression in human primary T cells.
Figures

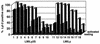


Similar articles
-
Effect of scaffold attachment region on transgene expression in retrovirus vector-transduced primary T cells and macrophages.Hum Gene Ther. 1999 May 20;10(8):1389-99. doi: 10.1089/10430349950018058. Hum Gene Ther. 1999. PMID: 10365668
-
RevM10-expressing T cells derived in vivo from transduced human hematopoietic stem-progenitor cells inhibit human immunodeficiency virus replication.J Virol. 1997 Jun;71(6):4707-16. doi: 10.1128/JVI.71.6.4707-4716.1997. J Virol. 1997. PMID: 9151864 Free PMC article.
-
Addition of the human interferon beta scaffold attachment region to retroviral vector backbones increases the level of in vivo transgene expression among progeny of engrafted human hematopoietic stem cells.Hum Gene Ther. 2000 Sep 20;11(14):2039-50. doi: 10.1089/10430340050143453. Hum Gene Ther. 2000. PMID: 11020802
-
High transdominant RevM10 protein levels are required to inhibit HIV-1 replication in cell lines and primary T cells: implication for gene therapy of AIDS.Gene Ther. 1997 Feb;4(2):128-39. doi: 10.1038/sj.gt.3300369. Gene Ther. 1997. PMID: 9081703
-
An improved vector for high-level, consistent retroviral transgene expression in human thymocytes after competitive reconstitution from transduced peripheral blood stem cells.Hum Gene Ther. 2001 Jul 1;12(10):1239-49. doi: 10.1089/104303401750270904. Hum Gene Ther. 2001. PMID: 11440618
Cited by
-
Effects of stuffer DNA on transgene expression from helper-dependent adenovirus vectors.J Virol. 1999 Oct;73(10):8027-34. doi: 10.1128/JVI.73.10.8027-8034.1999. J Virol. 1999. PMID: 10482551 Free PMC article.
-
A self-inactivating retrovector incorporating the IL-2 promoter for activation-induced transgene expression in genetically engineered T-cells.Virol J. 2006 Nov 21;3:97. doi: 10.1186/1743-422X-3-97. Virol J. 2006. PMID: 17118192 Free PMC article.
-
Construction and molecular analysis of gene transfer systems derived from bovine immunodeficiency virus.J Virol. 2001 Apr;75(7):3371-82. doi: 10.1128/JVI.75.7.3371-3382.2001. J Virol. 2001. PMID: 11238863 Free PMC article.
-
A chimeric HS4-SAR insulator (IS2) that prevents silencing and enhances expression of lentiviral vectors in pluripotent stem cells.PLoS One. 2014 Jan 6;9(1):e84268. doi: 10.1371/journal.pone.0084268. eCollection 2014. PLoS One. 2014. PMID: 24400083 Free PMC article.
-
T lymphocytes with a normal ADA gene accumulate after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates.Nat Med. 1998 Jul;4(7):775-80. doi: 10.1038/nm0798-775. Nat Med. 1998. PMID: 9662367 Free PMC article.
References
-
- Blaese R M, Culver K W, Miller A D, Carter C S, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt J J, Rosenberg S A, Klein H, Berger M, Mullen C A, Ramsey W J, Muul L, Morgan R A, Anderson W F. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science. 1995;270:475–480. - PubMed
-
- Bode J, Schlake T, Ríos-Ramírez M, Mielke C, Stengert M, Kay V, Klehr W D. Scaffold/matrix-attachment regions (S/MAR): structural properties creating transcriptionally active loci. Orlando, Fla: Academic Press, Inc.; 1995. - PubMed
-
- Bonyhadi M, Moss K, Voytovitch A, Auten J, Kalfoglou C, Plavec I, Forestell S, Su L, Böhnlein E, Kaneshima H. RevM10-expressing T cells derived in vivo from transduced human hematopoietic stem-progenitor cells inhibit human immunodeficiency virus replication. J Virol. 1997;71:4707–4716. - PMC - PubMed
-
- Bordignon C, Notarangelo L D, Nobili N, Ferrari G, Casorati G, Panina P, Mazzolari E, Maggioni D, Rossi C, Servida P, Ugazio A G, Mavilio F. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immundeficient patients. Science. 1995;270:470–475. - PubMed
-
- Boulikas T. Nature of DNA sequences at the attachment regions of genes to the nuclear matrix. J Cell Biochem. 1993;52:14–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

