Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription
- PMID: 9557655
- PMCID: PMC109595
- DOI: 10.1128/JVI.72.5.3729-3741.1998
Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription
Abstract
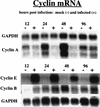
Human cytomegalovirus (HCMV) infection inhibits cell cycle progression and alters the expression of cyclins E, A, and B (F. M. Jault, J.-M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector, J. Virol. 69:6697-6704, 1995). In this study, we examined cell cycle progression, cyclin gene expression, and early viral events when the infection was initiated at different points in the cell cycle (G0, G1, and S). In all cases, infection led to cell cycle arrest. Cells infected in G0 or G1 phase also showed a complete or partial absence, respectively, of cellular DNA synthesis at a time when DNA synthesis occurred in the corresponding mock-infected cells. In contrast, when cells were infected near or during S phase, many cells were able to pass through S phase and undergo mitosis prior to cell cycle arrest. S-phase infection also produced a delay in the appearance of the viral cytopathic effect and in the synthesis of immediate-early and early proteins. Labeling of cells with bromodeoxyuridine immediately prior to HCMV infection in S phase revealed that viral protein expression occurred primarily in cells which were not engaged in DNA synthesis at the time of infection. The viral-mediated induction of cyclin E, maintenance of cyclin-B protein levels, and inhibitory effects on the accumulation of cyclin A were not significantly affected when infection occurred during different phases of the cell cycle (G0, G1, and S). However, there was a delay in the observed inhibition of cyclin A in cells infected during S phase. This finding was in accord with the pattern of cell cycle progression and delay in viral gene expression associated with S-phase infection. Analysis of the mRNA revealed that the effects of the virus on cyclin E and cyclin A, but not on cyclin B, were primarily at the transcriptional level.
Figures








Similar articles
-
Dysregulation of cyclin E gene expression in human cytomegalovirus-infected cells requires viral early gene expression and is associated with changes in the Rb-related protein p130.J Virol. 2000 May;74(9):4192-206. doi: 10.1128/jvi.74.9.4192-4206.2000. J Virol. 2000. PMID: 10756032 Free PMC article.
-
Mechanisms governing maintenance of Cdk1/cyclin B1 kinase activity in cells infected with human cytomegalovirus.J Virol. 2003 Dec;77(24):13214-24. doi: 10.1128/jvi.77.24.13214-13224.2003. J Virol. 2003. PMID: 14645578 Free PMC article.
-
The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation.EMBO J. 2001 Mar 1;20(5):1086-98. doi: 10.1093/emboj/20.5.1086. EMBO J. 2001. PMID: 11230132 Free PMC article.
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
Cited by
-
The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis.J Virol. 2004 Apr;78(8):4278-88. doi: 10.1128/jvi.78.8.4278-4288.2004. J Virol. 2004. PMID: 15047841 Free PMC article.
-
Human cytomegalovirus protein pUL117 targets the mini-chromosome maintenance complex and suppresses cellular DNA synthesis.PLoS Pathog. 2010 Mar 19;6(3):e1000814. doi: 10.1371/journal.ppat.1000814. PLoS Pathog. 2010. PMID: 20333247 Free PMC article.
-
Host-viral effects of chromatin assembly factor 1 interaction with HCMV IE2.Cell Res. 2011 Aug;21(8):1230-47. doi: 10.1038/cr.2011.53. Epub 2011 Mar 29. Cell Res. 2011. PMID: 21445097 Free PMC article.
-
Asparagine Deprivation Causes a Reversible Inhibition of Human Cytomegalovirus Acute Virus Replication.mBio. 2019 Oct 8;10(5):e01651-19. doi: 10.1128/mBio.01651-19. mBio. 2019. PMID: 31594813 Free PMC article.
-
Persistent HCMV infection of a glioblastoma cell line contributes to the development of resistance to temozolomide.Virus Res. 2020 Jan 15;276:197829. doi: 10.1016/j.virusres.2019.197829. Epub 2019 Nov 29. Virus Res. 2020. PMID: 31790777 Free PMC article.
References
-
- AbuBakar S, Au W W, Legator M S, Albrecht T. Induction of chromosome aberrations and mitotic arrest by cytomegalovirus in human cells. Environ Mol Mutagen. 1988;12:409–420. - PubMed
-
- Albrecht T, Boldogh I, Fons M, Lee C H, AbuBakar S, Russell J M, Au W W. Cell-activation responses to cytomegalovirus infection: relationship to the phasing of CMV replication and to the induction of cellular damage. Subcell Biochem. 1989;15:157–202. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1994.
-
- Beijersbergen R L, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

