Transgenic mice secreting coronavirus neutralizing antibodies into the milk
- PMID: 9557658
- PMCID: PMC109598
- DOI: 10.1128/JVI.72.5.3762-3772.1998
Transgenic mice secreting coronavirus neutralizing antibodies into the milk
Abstract
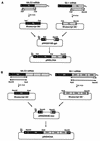
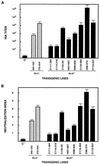
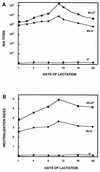
Ten lines of transgenic mice secreting transmissible gastroenteritis coronavirus (TGEV) neutralizing recombinant monoclonal antibodies (rMAbs) into the milk were generated. The rMAb light- and heavy-chain genes were assembled by fusing the genes encoding the variable modules of the murine MAb 6A.C3, which binds an interspecies conserved coronavirus epitope essential for virus infectivity, and a constant module from a porcine myeloma with the immunoglobulin A (IgA) isotype. The chimeric antibody led to dimer formation in the presence of J chain. The neutralization specific activity of the recombinant antibody produced in transiently or stably transformed cells was 50-fold higher than that of a monomeric rMAb with the IgG1 isotype and an identical binding site. This rMAb had titers of up to 10(4) by radioimmunoassay (RIA) and neutralized virus infectivity up to 10(4)-fold. Of 23 transgenic mice, 17 integrated both light and heavy chains, and at least 10 of them transmitted both genes to the progeny, leading to 100% of animals secreting functional TGEV neutralizing antibody during lactation. Selected mice produced milk with TGEV-specific antibody titers higher than 10(6) as determined by RIA, neutralized virus infectivity by 10(6)-fold, and produced up to 6 mg of antibody per ml. Antibody expression levels were transgene copy number independent and integration site dependent. Comicroinjection of the genomic beta-lactoglobulin gene with rMAb light- and heavy-chain genes led to the generation of transgenic mice carrying the three transgenes. The highest antibody titers were produced by transgenic mice that had integrated the antibody and beta-lactoglobulin genes, although the number of transgenic animals generated does not allow a definitive conclusion on the enhancing effect of beta-lactoglobulin cointegration. This approach may lead to the generation of transgenic animals providing lactogenic immunity to their progeny against enteric pathogens.
Figures



References
-
- Ali S, Clark A J. Characterization of the gene encoding ovine β-lactoglobulin. J Mol Biol. 1988;199:415–426. - PubMed
-
- Ali S, McClenaghan M, Simons J P, Clark A J. Characterisation of the alleles encoding ovine β-lactoglobulins A and B. Gene. 1990;91:201–207. - PubMed
-
- Armstrong S J, Outlaw M C, Dimmock N J. Morphological studies of neutralization of influenza virus by IgM. J Gen Virol. 1990;71:2313–2319. - PubMed
-
- Brim T A, VanCott J L, Lunney J K, Saif L J. Lymphocyte proliferation responses of pigs inoculated with transmissible gastroenteritis virus or porcine respiratory coronavirus. Am J Vet Res. 1994;55:494–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

