Two RING finger proteins, the oncoprotein PML and the arenavirus Z protein, colocalize with the nuclear fraction of the ribosomal P proteins
- PMID: 9557665
- PMCID: PMC109605
- DOI: 10.1128/JVI.72.5.3819-3826.1998
Two RING finger proteins, the oncoprotein PML and the arenavirus Z protein, colocalize with the nuclear fraction of the ribosomal P proteins
Abstract
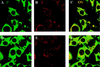
The promyelocytic leukemia (PML) protein forms nuclear bodies which are relocated to the cytoplasm by the RNA virus lymphocytic choriomeningitis virus (LCMV). The viral Z protein directly binds to PML and can relocate the nuclear bodies. Others have observed that LCMV virions may contain ribosomes; hence, we investigated the effects of infection on the distribution of ribosomal P proteins (P0, P1, and P2) with PML as a reference point. We demonstrate an association of PML bodies with P proteins by indirect immunofluorescence and coimmunoprecipitation experiments, providing the first evidence of nucleic acid-binding proteins associated with PML bodies. We show that unlike PML, the P proteins are not redistributed upon infection. Immunofluorescence and coimmunoprecipitation studies indicate that the viral Z protein binds the nuclear, but not the cytoplasmic, fraction of P0. The nuclear fraction of P0 has been associated with translationally coupled DNA excision repair and with nonspecific endonuclease activity; thus, P0 may be involved in nucleic acid processing activities necessary for LCMV replication. During the infection process, PML, P1, and P2 are downregulated but P0 remains unchanged. Further, P0 is present in virions while PML is not, indicating some selectivity in the assembly of LCMV.
Figures




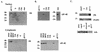
References
-
- Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. PIC1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene. 1996;13:971–982. - PubMed
-
- Borden K L B, Freemont P S. The RING finger: an example of a sequence structure family. Curr Opin Struct Biol. 1996;6:395–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

