Hepatitis C virus structural proteins assemble into viruslike particles in insect cells
- PMID: 9557666
- PMCID: PMC109606
- DOI: 10.1128/JVI.72.5.3827-3836.1998
Hepatitis C virus structural proteins assemble into viruslike particles in insect cells
Abstract
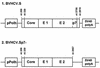
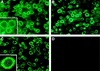
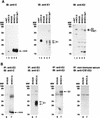
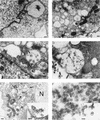
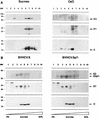
Hepatitis C virus (HCV) is a leading cause of chronic hepatitis in the world. The study of HCV has been hampered by the low level of viral particles in infected individuals, the inability to propagate efficiently the virus in cultured cells, and the lack of a convenient animal model. Due to these obstacles, neither the structure of the virus nor the prerequisites for its assembly have been clearly defined. In this report, we describe a model for the production and purification of HCV-like particles in insect cells using a recombinant baculovirus containing the cDNA of the HCV structural proteins. In insect cells, expressed HCV structural proteins assembled into enveloped viruslike particles (40 to 60 nm in diameter) in large cytoplasmic cisternae, presumably derived from the endoplasmic reticulum. Biophysical characterization of viruslike particles by CsCl and sucrose gradient centrifugation revealed biophysical properties similar to those of putative virions isolated from infected humans. The results suggested that HCV core and envelope proteins without p7 were sufficient for viral particle formation. Analysis of particle-associated nucleic acids demonstrated that HCV RNAs were selectively incorporated into the particles over non-HCV transcripts. The synthesis of HCV-like particles in insect cells may provide an important tool to determine the structural requirements for HCV particle assembly as well as to study viral genome encapsidation and virus-host interactions. The described system may also represent a potential approach toward vaccine development.
Figures
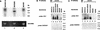
References
-
- Alter H J, Purcell R H, Shih J W, Melpolder J C, Houghton M, Choo Q-L, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. - PubMed
-
- Bielefeldt Ohmann H, Bloch B. Electron microscopic studies of bovine viral diarrhea virus in tissues of diseased calves and in cell cultures. Arch Virol. 1981;71:57–74. - PubMed
-
- Chomczynski P N, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

