Carbohydrates facilitate correct disulfide bond formation and folding of rotavirus VP7
- PMID: 9557673
- PMCID: PMC109613
- DOI: 10.1128/JVI.72.5.3887-3892.1998
Carbohydrates facilitate correct disulfide bond formation and folding of rotavirus VP7
Abstract
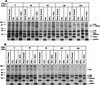
It is well established that glycosylation is essential for assembly of enveloped viruses, but no information is yet available as to the function of carbohydrates on the nonenveloped but glycosylated rotavirus. We show that tunicamycin and, more pronouncedly, a combination of tunicamycin and brefeldin A treatment caused misfolding of the luminal VP7 protein, leading to interdisulfide bond aggregation. While formation of VP7 aggregates could be prevented under reducing conditions, they reoccurred in less than 30 min after a shift to an oxidizing milieu. Furthermore, while glycosylated VP7 interacted during maturation with protein disulfide isomerase, nonglycosylated VP7 did not, suggesting that glycosylation is a prerequisite for protein disulfide isomerase interaction. While native NSP4, which does not possess S-S bonds, was not dependent on N-linked glycosylation or on protein disulfide isomerase assistance for maturation, nonglycosylated NSP4 was surprisingly found to interact with protein disulfide isomerase, further suggesting that protein disulfide isomerase can act both as an enzyme and as a chaperone. In conclusion, our data suggest that the major function of carbohydrates on VP7 is to facilitate correct disulfide bond formation and protein folding.
Figures

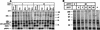
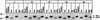

Similar articles
-
The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro.J Virol. 1998 Nov;72(11):8705-9. doi: 10.1128/JVI.72.11.8705-8709.1998. J Virol. 1998. PMID: 9765412 Free PMC article.
-
ATP is required for correct folding and disulfide bond formation of rotavirus VP7.J Virol. 2000 Sep;74(17):8048-52. doi: 10.1128/jvi.74.17.8048-8052.2000. J Virol. 2000. PMID: 10933714 Free PMC article.
-
Intracellular manipulation of disulfide bond formation in rotavirus proteins during assembly.J Virol. 1994 Aug;68(8):5204-15. doi: 10.1128/JVI.68.8.5204-5215.1994. J Virol. 1994. PMID: 8035518 Free PMC article.
-
PDI-Regulated Disulfide Bond Formation in Protein Folding and Biomolecular Assembly.Molecules. 2020 Dec 31;26(1):171. doi: 10.3390/molecules26010171. Molecules. 2020. PMID: 33396541 Free PMC article. Review.
-
A hypothesis about the mechanism of assembly of double-shelled rotavirus particles.Arch Virol Suppl. 1996;12:79-85. doi: 10.1007/978-3-7091-6553-9_9. Arch Virol Suppl. 1996. PMID: 9015104 Review.
Cited by
-
The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro.J Virol. 1998 Nov;72(11):8705-9. doi: 10.1128/JVI.72.11.8705-8709.1998. J Virol. 1998. PMID: 9765412 Free PMC article.
-
Inflammatory and oxidative stress in rotavirus infection.World J Virol. 2016 May 12;5(2):38-62. doi: 10.5501/wjv.v5.i2.38. World J Virol. 2016. PMID: 27175349 Free PMC article. Review.
-
Spike protein VP4 assembly with maturing rotavirus requires a postendoplasmic reticulum event in polarized caco-2 cells.J Virol. 2004 Oct;78(20):10987-94. doi: 10.1128/JVI.78.20.10987-10994.2004. J Virol. 2004. PMID: 15452219 Free PMC article.
-
Hidden Relationships between N-Glycosylation and Disulfide Bonds in Individual Proteins.Int J Mol Sci. 2022 Mar 29;23(7):3742. doi: 10.3390/ijms23073742. Int J Mol Sci. 2022. PMID: 35409101 Free PMC article. Review.
-
Modeling of the rotavirus group C capsid predicts a surface topology distinct from other rotavirus species.Virology. 2016 Jan;487:150-62. doi: 10.1016/j.virol.2015.10.017. Epub 2015 Nov 2. Virology. 2016. PMID: 26524514 Free PMC article.
References
-
- Ball J M, Tian P, Zeng C Q, Morris A P, Estes M K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. . (See comments.) - PubMed
-
- Cai H, Wang C C, Tsou C L. Chaperone-like activity of protein disulfide isomerase in the refolding of a protein with no disulfide bonds. J Biol Chem. 1994;269:24550–24552. - PubMed
-
- Chan W K, Au K S, Estes M K. Topography of the simian rotavirus nonstructural glycoprotein (NS28) in the endoplasmic reticulum membrane. Virology. 1988;164:435–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

