Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure
- PMID: 9557706
- PMCID: PMC109646
- DOI: 10.1128/JVI.72.5.4170-4182.1998
Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure
Abstract
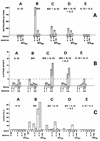
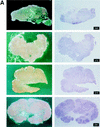
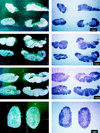
Vaccine protection from infection and/or disease induced by highly pathogenic simian immunodeficiency virus (SIV) strain SIV(mac251) in the rhesus macaque model is a challenging task. Thus far, the only approach that has been reported to protect a fraction of macaques from infection following intravenous challenge with SIV(mac251) was the use of a live attenuated SIV vaccine. In the present study, the gag, pol, and env genes of SIV(K6W) were expressed in the NYVAC vector, a genetically engineered derivative of the vaccinia virus Copenhagen strain that displays a highly attenuated phenotype in humans. In addition, the genes for the alpha and beta chains of interleukin-12 (IL-12), as well as the IL-2 gene, were expressed in separate NYVAC vectors and inoculated intramuscularly, in conjunction with or separate from the NYVAC-SIV vaccine, in 40 macaques. The overall cytotoxic T-lymphocyte (CTL) response was greater, at the expense of proliferative and humoral responses, in animals immunized with NYVAC-SIV and NYVAC-IL-12 than in animals immunized with the NYVAC-SIV vaccine alone. At the end of the immunization regimen, half of the animals were challenged with SIV(mac251) by the intravenous route and the other half were exposed to SIV(mac251) intrarectally. Significantly, five of the eleven vaccinees exposed mucosally to SIV(mac251) showed a transient peak of viremia 1 week after viral challenge and subsequently appeared to clear viral infection. In contrast, all 12 animals inoculated intravenously became infected, but 5 to 6 months after viral challenge, 4 animals were able to control viral expression and appeared to progress to disease more slowly than control animals. Protection did not appear to be associated with any of the measured immunological parameters. Further modulation of immune responses by coadministration of NYVAC-cytokine recombinants did not appear to influence the outcome of viral challenge. The fact that the NYVAC-SIV recombinant vaccine appears to be effective per se in the animal model that best mirrors human AIDS supports the idea that the development of a highly attenuated poxvirus-based vaccine candidate can be a valuable approach to significantly decrease the spread of human immunodeficiency virus (HIV) infection by the mucosal route.
Figures
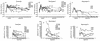






References
-
- Abimiku A G, Franchini G, Tartaglia J, Aldrich K, Myagkikh M, Markham P D, Chong P, Klein M, Kieny M P, Paoletti E, Gallo R C, Robert-Guroff M. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med. 1995;1:321–329. - PubMed
-
- Abimiku A G, Robert-Guroff M, Benson J, Tartaglia J, Paoletti E, Gallo R C, Markham P D, Franchini G. Long-term survival of SIVmac251-infected macaques previously immunized with NYVAC-SIV vaccines. J Acquired Immune Defic Syndr. 1997;15:S78–S85.
-
- Afonso L C C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235. - PubMed
-
- Andersson S, Makitalo B, Thorstensson R, Franchini G, Tartaglia J, Limbach K, Paoletti E, Putkonen P, Biberfeld G. Immunogenicity and protective efficacy of an HIV-2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J Infect Dis. 1996;174:977–985. - PubMed
-
- Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

